当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Selective Targeting of Guanine-Vacancy-Bearing G-Quadruplexes by G-Quartet Complementation and Stabilization with a Guanine-Peptide Conjugate
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-06-03 , DOI: 10.1021/jacs.0c00774
Yi-de He 1 , Ke-Wei Zheng 1 , Cui-Jiao Wen 1 , Xin-Min Li 1 , Jia-Yuan Gong 1 , Yu-Hua Hao 1 , Yong Zhao , Zheng Tan 1, 2
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-06-03 , DOI: 10.1021/jacs.0c00774
Yi-de He 1 , Ke-Wei Zheng 1 , Cui-Jiao Wen 1 , Xin-Min Li 1 , Jia-Yuan Gong 1 , Yu-Hua Hao 1 , Yong Zhao , Zheng Tan 1, 2
Affiliation
|
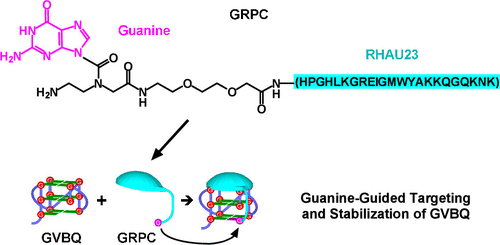
Stabilization of G-quadruplexes (G4s) formed in guanine-rich (G-rich) nucleic acids by small-molecule ligands has been extensively explored as a therapeutic approach for diseases such as cancer. Finding ligands with sufficient affinity and specificity towards G4s remains a challenge and many ligands reported seemed to compromise between the two features. To cope with this challenge, we focused on targeting a particular type of G4s, i.e. the G-vacancy-bearing G-quadruplexes (GVBQs), by taking a structure complementation strategy to enhance both affinity and selectivity. In this approach, a G-quadruplex-binding peptide RHAU23 is guided toward a GVBQ by a guanine moiety covalently linked to the peptide. The filling-in of the vacancy in a GVBQ by the guanine ensures an exclusive recognition of GVBQ. Moreover, the synergy between the RHAU23 and the guanine dramatically improves both the affinity towards and stabilization of the GVBQ. Targeting a GVBQ in DNA by this bi-functional peptide strongly suppresses in vitro replication. This study demonstrates a novel and promising alternative targeting strategy to a distinctive panel of G4s that are as abundant as the canonical ones in the human genome.
中文翻译:

用鸟嘌呤-肽缀合物通过 G-四重体互补和稳定来选择性靶向带有鸟嘌呤空位的 G-四链体
小分子配体在富含鸟嘌呤(富含 G)的核酸中形成的 G-四链体(G4s)的稳定性已被广泛探索作为治疗癌症等疾病的方法。寻找对 G4s 具有足够亲和力和特异性的配体仍然是一个挑战,许多报道的配体似乎在这两个特征之间妥协。为了应对这一挑战,我们通过采用结构互补策略来增强亲和力和选择性,专注于针对特定类型的 G4,即带有 G 空位的 G-四链体 (GVBQ)。在这种方法中,G-四链体结合肽 RHAU23 通过与肽共价连接的鸟嘌呤部分被引导至 GVBQ。鸟嘌呤填补 GVBQ 中的空缺确保了 GVBQ 的独家认可。而且,RHAU23 和鸟嘌呤之间的协同作用显着提高了 GVBQ 的亲和力和稳定性。通过这种双功能肽靶向 DNA 中的 GVBQ 可强烈抑制体外复制。这项研究展示了一种新颖且有前景的替代靶向策略,用于与人类基因组中的规范基因一样丰富的一组独特的 G4。
更新日期:2020-06-03
中文翻译:

用鸟嘌呤-肽缀合物通过 G-四重体互补和稳定来选择性靶向带有鸟嘌呤空位的 G-四链体
小分子配体在富含鸟嘌呤(富含 G)的核酸中形成的 G-四链体(G4s)的稳定性已被广泛探索作为治疗癌症等疾病的方法。寻找对 G4s 具有足够亲和力和特异性的配体仍然是一个挑战,许多报道的配体似乎在这两个特征之间妥协。为了应对这一挑战,我们通过采用结构互补策略来增强亲和力和选择性,专注于针对特定类型的 G4,即带有 G 空位的 G-四链体 (GVBQ)。在这种方法中,G-四链体结合肽 RHAU23 通过与肽共价连接的鸟嘌呤部分被引导至 GVBQ。鸟嘌呤填补 GVBQ 中的空缺确保了 GVBQ 的独家认可。而且,RHAU23 和鸟嘌呤之间的协同作用显着提高了 GVBQ 的亲和力和稳定性。通过这种双功能肽靶向 DNA 中的 GVBQ 可强烈抑制体外复制。这项研究展示了一种新颖且有前景的替代靶向策略,用于与人类基因组中的规范基因一样丰富的一组独特的 G4。

































 京公网安备 11010802027423号
京公网安备 11010802027423号