当前位置:
X-MOL 学术
›
J. Electroanal. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cobalt ferrite nanoparticles as a source of intrinsic metals for simultaneous electrosynthesis of Prussian blue and cobalt hexacyanoferrate
Journal of Electroanalytical Chemistry ( IF 4.1 ) Pub Date : 2020-08-01 , DOI: 10.1016/j.jelechem.2020.114315 Nielson José Silva Furtado , Janildo Lopes Magalhães
Journal of Electroanalytical Chemistry ( IF 4.1 ) Pub Date : 2020-08-01 , DOI: 10.1016/j.jelechem.2020.114315 Nielson José Silva Furtado , Janildo Lopes Magalhães
|
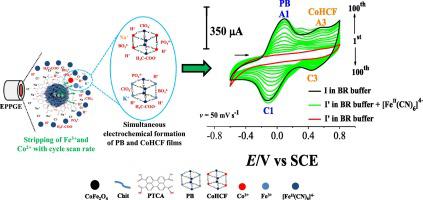
Abstract A novel method is proposed for the simultaneous electrochemical formation of Prussian blue (PB) and cobalt hexacyanoferrate (CoHCF) on the surface of edge-plane pyrolytic graphite electrodes (EPPGE) modified by CoFe2O4/Chit/PTCA nanoparticle (NP) (where CoFe2O4 = cobalt ferrite, Chit = chitosan, and PTCA = Perylene-3,4,9,10-tetracarboxylic acid). The PB and CoHCF films are formed from the NP intrinsic metals (Fe3+ and Co2+) in the presence of ferrocyanide ([FeII(CN)6]4−) dissolved in a Britton-Robinson (BR) buffer at pH 1.60. The extent of the film formation is determined by the amount of the PTCA immobilised on the CoFe2O4/Chit NP surface and is influenced by the components of the BR buffer medium. Larger amounts of immobilised PTCA enhance electrosynthesis and generate modified electrodes in situ with excellent electrochemical properties. Optimized electrodes increased electroactive area approximately 420% compared to bare EPPGE. We believe that the combination of NP intrinsic metals, PTCA immobilisation, use of [FeII(CN)6]4− and BR buffer components provides a new electrochemical structure for simultaneous production of PB and CoHCF films. The quality of the electrodes generated in situ by the present method is evidenced by their increased sensitivity and enhanced active surface area relative to bare EPPGE, which indicates its potential for future application in electrochemical sensors.
中文翻译:

铁氧体钴纳米粒子作为同时电合成普鲁士蓝和六氰基铁酸钴的固有金属来源
摘要 提出了一种在 CoFe2O4/Chit/PTCA 纳米粒子 (NP) 修饰的边平面热解石墨电极 (EPPGE) 表面同时电化学形成普鲁士蓝 (PB) 和六氰基铁酸钴 (CoHCF) 的新方法(其中 CoFe2O4 = 铁酸钴,Chit = 壳聚糖,PTCA = 苝-3,4,9,10-四羧酸)。PB 和 CoHCF 薄膜由 NP 本征金属(Fe3+ 和 Co2+)在亚铁氰化物([FeII(CN)6]4-)存在下溶解在 pH 1.60 的 Britton-Robinson (BR) 缓冲液中形成。成膜程度由固定在 CoFe2O4/Chit NP 表面的 PTCA 量决定,并受 BR 缓冲介质组分的影响。大量固定化 PTCA 可增强电合成并原位生成具有优异电化学性能的修饰电极。与裸 EPPGE 相比,优化的电极增加了大约 420% 的电活性面积。我们认为,NP 固有金属、PTCA 固定化、[FeII(CN)6]4- 和 BR 缓冲组分的使用为同时生产 PB 和 CoHCF 薄膜提供了一种新的电化学结构。通过本方法原位生成的电极的质量通过相对于裸 EPPGE 增加的灵敏度和增强的活性表面积来证明,这表明其在电化学传感器中的未来应用潜力。PTCA 固定、[FeII(CN)6]4- 和 BR 缓冲组分的使用为同时生产 PB 和 CoHCF 薄膜提供了一种新的电化学结构。通过本方法原位生成的电极的质量通过相对于裸 EPPGE 增加的灵敏度和增强的活性表面积来证明,这表明其在电化学传感器中的未来应用潜力。PTCA 固定、[FeII(CN)6]4- 和 BR 缓冲组分的使用为同时生产 PB 和 CoHCF 薄膜提供了一种新的电化学结构。通过本方法原位生成的电极的质量通过相对于裸 EPPGE 增加的灵敏度和增强的活性表面积来证明,这表明其在电化学传感器中的未来应用潜力。
更新日期:2020-08-01
中文翻译:

铁氧体钴纳米粒子作为同时电合成普鲁士蓝和六氰基铁酸钴的固有金属来源
摘要 提出了一种在 CoFe2O4/Chit/PTCA 纳米粒子 (NP) 修饰的边平面热解石墨电极 (EPPGE) 表面同时电化学形成普鲁士蓝 (PB) 和六氰基铁酸钴 (CoHCF) 的新方法(其中 CoFe2O4 = 铁酸钴,Chit = 壳聚糖,PTCA = 苝-3,4,9,10-四羧酸)。PB 和 CoHCF 薄膜由 NP 本征金属(Fe3+ 和 Co2+)在亚铁氰化物([FeII(CN)6]4-)存在下溶解在 pH 1.60 的 Britton-Robinson (BR) 缓冲液中形成。成膜程度由固定在 CoFe2O4/Chit NP 表面的 PTCA 量决定,并受 BR 缓冲介质组分的影响。大量固定化 PTCA 可增强电合成并原位生成具有优异电化学性能的修饰电极。与裸 EPPGE 相比,优化的电极增加了大约 420% 的电活性面积。我们认为,NP 固有金属、PTCA 固定化、[FeII(CN)6]4- 和 BR 缓冲组分的使用为同时生产 PB 和 CoHCF 薄膜提供了一种新的电化学结构。通过本方法原位生成的电极的质量通过相对于裸 EPPGE 增加的灵敏度和增强的活性表面积来证明,这表明其在电化学传感器中的未来应用潜力。PTCA 固定、[FeII(CN)6]4- 和 BR 缓冲组分的使用为同时生产 PB 和 CoHCF 薄膜提供了一种新的电化学结构。通过本方法原位生成的电极的质量通过相对于裸 EPPGE 增加的灵敏度和增强的活性表面积来证明,这表明其在电化学传感器中的未来应用潜力。PTCA 固定、[FeII(CN)6]4- 和 BR 缓冲组分的使用为同时生产 PB 和 CoHCF 薄膜提供了一种新的电化学结构。通过本方法原位生成的电极的质量通过相对于裸 EPPGE 增加的灵敏度和增强的活性表面积来证明,这表明其在电化学传感器中的未来应用潜力。












































 京公网安备 11010802027423号
京公网安备 11010802027423号