当前位置:
X-MOL 学术
›
J. Mol. Struct.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Galantamine derivatives: Synthesis, NMR study, DFT calculations and application in asymmetric catalysis
Journal of Molecular Structure ( IF 4.0 ) Pub Date : 2020-11-01 , DOI: 10.1016/j.molstruc.2020.128568 Irena Philipova , Georgi Stavrakov , Vladimir Dimitrov , Nikolay Vassilev
Journal of Molecular Structure ( IF 4.0 ) Pub Date : 2020-11-01 , DOI: 10.1016/j.molstruc.2020.128568 Irena Philipova , Georgi Stavrakov , Vladimir Dimitrov , Nikolay Vassilev
|
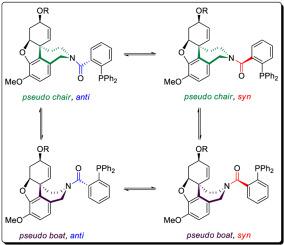
Abstract In a search of effective ligands for asymmetric catalysis (−)-galantamine has been selected as a complex chiral framework for the synthesis of four novel diphenylphosphino-benzenecarboxamides. Their application in Pd-catalyzed asymmetric allylic alkylation proceeded with excellent conversion and moderate enantioselectivity due to the conformational flexibility of the galantamine derived compounds. To get insights into their molecular structure and conformational behaviour in solution a combination of experimental NMR methods and theoretical DFT calculations has been employed. The ligands exist as four conformers due to restricted rotation around the amide bond and due to flexibility of the 2,3,4,5-tetrahydro-1H-azepine ring. The experimentally measured barriers of C–N rotation (17.1 ÷ 17.7 kcal/mol) are higher than the barriers of observed exchange process in azepine ring (13.7 ÷ 14.0 kcal/mol). Their BOC precursors exist in solution as two conformers due to restricted rotation around the carbamate C–N bond. The experimentally measured barrier is lower than the amide barriers in ligands (16.1 ÷ 16.5 kcal/mol).
中文翻译:

加兰他敏衍生物:合成、核磁共振研究、DFT 计算和在不对称催化中的应用
摘要 为了寻找不对称催化的有效配体,(-)-加兰他敏已被选为合成四种新型二苯基膦基-苯甲酰胺的复杂手性框架。由于加兰他敏衍生化合物的构象灵活性,它们在 Pd 催化的不对称烯丙基烷基化中的应用具有出色的转化率和适度的对映选择性。为了深入了解它们在溶液中的分子结构和构象行为,结合了实验 NMR 方法和理论 DFT 计算。由于围绕酰胺键的旋转受限以及 2,3,4,5-四氢-1H-氮杂环的柔韧性,配体以四个构象异构体形式存在。实验测量的 C-N 旋转障碍(17.1 ÷ 17. 7 kcal/mol) 高于观察到的氮杂环交换过程的势垒 (13.7 ÷ 14.0 kcal/mol)。由于围绕氨基甲酸酯 C-N 键的旋转受限,它们的 BOC 前体作为两个构象异构体存在于溶液中。实验测量的屏障低于配体中的酰胺屏障 (16.1 ÷ 16.5 kcal/mol)。
更新日期:2020-11-01
中文翻译:

加兰他敏衍生物:合成、核磁共振研究、DFT 计算和在不对称催化中的应用
摘要 为了寻找不对称催化的有效配体,(-)-加兰他敏已被选为合成四种新型二苯基膦基-苯甲酰胺的复杂手性框架。由于加兰他敏衍生化合物的构象灵活性,它们在 Pd 催化的不对称烯丙基烷基化中的应用具有出色的转化率和适度的对映选择性。为了深入了解它们在溶液中的分子结构和构象行为,结合了实验 NMR 方法和理论 DFT 计算。由于围绕酰胺键的旋转受限以及 2,3,4,5-四氢-1H-氮杂环的柔韧性,配体以四个构象异构体形式存在。实验测量的 C-N 旋转障碍(17.1 ÷ 17. 7 kcal/mol) 高于观察到的氮杂环交换过程的势垒 (13.7 ÷ 14.0 kcal/mol)。由于围绕氨基甲酸酯 C-N 键的旋转受限,它们的 BOC 前体作为两个构象异构体存在于溶液中。实验测量的屏障低于配体中的酰胺屏障 (16.1 ÷ 16.5 kcal/mol)。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号