当前位置:
X-MOL 学术
›
Chem. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Unusual Atmospheric Water Trapping and Water Induced Reversible Restacking of 2D Gallium Sulfide Layers in NaGaS2 Formed by Supertetrahedral Building Unit
Chemistry of Materials ( IF 7.2 ) Pub Date : 2020-06-01 , DOI: 10.1021/acs.chemmater.0c00836 Amit Adhikary 1 , Hooman Yaghoobnejad Asl 1 , Prashanth Sandineni 1 , Srikanth Balijapelly 1 , Sudip Mohapatra 1 , Sajal Khatua 2 , Sanjit Konar 2 , Nikolay Gerasimchuk 3 , Aleksandr V. Chernatynskiy 4 , Amitava Choudhury 1
Chemistry of Materials ( IF 7.2 ) Pub Date : 2020-06-01 , DOI: 10.1021/acs.chemmater.0c00836 Amit Adhikary 1 , Hooman Yaghoobnejad Asl 1 , Prashanth Sandineni 1 , Srikanth Balijapelly 1 , Sudip Mohapatra 1 , Sajal Khatua 2 , Sanjit Konar 2 , Nikolay Gerasimchuk 3 , Aleksandr V. Chernatynskiy 4 , Amitava Choudhury 1
Affiliation
|
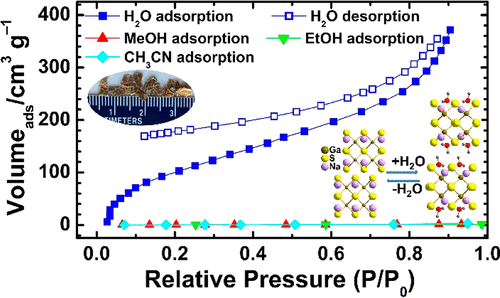
Two new ternary materials NaGaS2 (1) and the Fe-doped phase of NaGaS2, NaFe0.135Ga0.865S2 (2), have been synthesized by employing polysulfide flux. Single crystal XRD analyses of 1 and 2 show that the structure is built up of adamantane-like Ga4S10 super tetrahedral fundamental building units. These admantane-like units are connected through their corners to form [GaS2]∞– layers that are stacked one over the other with Na ions residing in between the layers to balance the charge. Both the materials have the remarkable ability to absorb atmospheric water molecules and moisture from undried solvents as verified by TG analysis and FT-IR and XPS studies. The process of water absorption leads to stable distinct material NaGaS2·∼H2O (1·H2O) and NaFe0.135Ga0.865S2·∼H2O (2·H2O) with restacked layers different from original crystal structure. This structural transformation is reversible as the transformed structures 1·H2O and 2·H2O can be returned to their original structures 1 and 2, respectively, upon heating. DFT calculation study reveals that a spontaneous exergonic hydration reaction takes place as outlined in NaGaS2 + H2O → NaGaS2·H2O with the energy release, ΔE of −73.9 kJ mol–1. DFT calculation predicts an increase in the unit cell parameters of b and c directions and shrinkage along the a direction of hydrated phase 1·H2O with an overall volume increase of 36.6%. Structural transformation affects their physical properties as the pristine compound 1 possess Na+ ion conductivity of 2.88 × 10–7 S cm–1 at 22 °C, whereas the hydrated compound 1·H2O displays ∼40 times increased ion conductivity of 1.25 × 10–5 S cm–1 at the same temperature. DRS studies show very similar optical band gaps of ∼4 eV for compounds 1 and 1·H2O, respectively, in reasonable agreement with the DFT(HSE) band gap estimation but more than 1 eV above the DFT(PBE)-predicted band gaps of ∼2.4 eV. A sorption study indicates selective adsorption of water over MeOH, EtOH, and CH3CN with a maximum water uptake of 2.6 H2O per formula unit at P/P0 = 0.9. A Karl Fischer titration study shows that NaGaS2 (1) is certainly capable of adsorbing water from wet methanol and can be useful as a fast desiccating agent.
中文翻译:

超四面体构建单元形成的NaGaS 2中二维硫化镓层的异常大气水截留和水诱导的可逆性重堆积
两个新的三元材料纳迦2(1)和纳迦的Fe掺杂阶段2,NaFe 0.135嘎0.865 š 2(2),已经通过采用多硫化物助熔剂合成。对1和2的单晶XRD分析表明,该结构由类似金刚烷的Ga 4 S 10超四面体基本建筑单元组成。这些金刚烷状单元通过其转角连接,以形成[气体2 ] ∞ -一层一层又一层地堆叠在一起,而Na离子则位于两层之间以平衡电荷。TG分析,FT-IR和XPS研究证明,这两种材料均具有从未干燥溶剂吸收大气水分子和水分的出色能力。吸水过程产生稳定的独特材料NaGaS 2 ·〜H 2 O(1 ·H 2 O)和NaFe 0.135 Ga 0.865 S 2 ·〜H 2 O(2 ·H 2 O),其重堆积层不同于原始晶体结构体。这种结构转化是可逆的,因为转化的结构为1 ·H2 O和2 ·H 2 O在加热时可以分别返回其原始结构1和2。DFT计算研究表明,如NaGaS 2 + H 2 O→NaGaS 2 ·H 2 O所述,发生了自发的能级水合反应,能量释放ΔE为-73.9 kJ mol –1。DFT计算预测b和c方向的晶胞参数增加,并且沿水合相1 ·H 2的a方向收缩。O总量增加了36.6%。结构转变影响它们的物理性质,因为原始化合物1在22°C下的Na +离子电导率为2.88×10 –7 S cm –1,而水合化合物1 ·H 2 O的离子电导率为1.25×约40倍。在相同温度下为10 –5 S cm –1。DRS研究表明,化合物1和1 ·H 2的光学带隙非常相似,约为〜4 eVO分别与DFT(HSE)带隙估计合理吻合,但比DFT(PBE)预测的〜2.4 eV带隙高1 eV以上。吸附研究表明的水选择性吸附在MeOH中,EtOH和CH 3 CN 2.6 H的最大水吸收2在澳每公式单位P / P 0 = 0.9。卡尔·费休滴定研究表明,NaGaS 2(1)当然能够从湿甲醇中吸附水,并且可用作快速干燥剂。
更新日期:2020-07-14
中文翻译:

超四面体构建单元形成的NaGaS 2中二维硫化镓层的异常大气水截留和水诱导的可逆性重堆积
两个新的三元材料纳迦2(1)和纳迦的Fe掺杂阶段2,NaFe 0.135嘎0.865 š 2(2),已经通过采用多硫化物助熔剂合成。对1和2的单晶XRD分析表明,该结构由类似金刚烷的Ga 4 S 10超四面体基本建筑单元组成。这些金刚烷状单元通过其转角连接,以形成[气体2 ] ∞ -一层一层又一层地堆叠在一起,而Na离子则位于两层之间以平衡电荷。TG分析,FT-IR和XPS研究证明,这两种材料均具有从未干燥溶剂吸收大气水分子和水分的出色能力。吸水过程产生稳定的独特材料NaGaS 2 ·〜H 2 O(1 ·H 2 O)和NaFe 0.135 Ga 0.865 S 2 ·〜H 2 O(2 ·H 2 O),其重堆积层不同于原始晶体结构体。这种结构转化是可逆的,因为转化的结构为1 ·H2 O和2 ·H 2 O在加热时可以分别返回其原始结构1和2。DFT计算研究表明,如NaGaS 2 + H 2 O→NaGaS 2 ·H 2 O所述,发生了自发的能级水合反应,能量释放ΔE为-73.9 kJ mol –1。DFT计算预测b和c方向的晶胞参数增加,并且沿水合相1 ·H 2的a方向收缩。O总量增加了36.6%。结构转变影响它们的物理性质,因为原始化合物1在22°C下的Na +离子电导率为2.88×10 –7 S cm –1,而水合化合物1 ·H 2 O的离子电导率为1.25×约40倍。在相同温度下为10 –5 S cm –1。DRS研究表明,化合物1和1 ·H 2的光学带隙非常相似,约为〜4 eVO分别与DFT(HSE)带隙估计合理吻合,但比DFT(PBE)预测的〜2.4 eV带隙高1 eV以上。吸附研究表明的水选择性吸附在MeOH中,EtOH和CH 3 CN 2.6 H的最大水吸收2在澳每公式单位P / P 0 = 0.9。卡尔·费休滴定研究表明,NaGaS 2(1)当然能够从湿甲醇中吸附水,并且可用作快速干燥剂。































 京公网安备 11010802027423号
京公网安备 11010802027423号