当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Adsorbent–Adsorbate Interactions in the Oxidation of HMF Catalyzed by Ni-Based MOFs: A DRIFT and FT-IR Insight
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2016-07-07 00:00:00 , DOI: 10.1021/acs.jpcc.6b05428
Carlo Lucarelli 1, 2 , Simona Galli 1, 2 , Angelo Maspero 1 , Alessandro Cimino 1 , Claudia Bandinelli 3 , Alice Lolli 3 , Juliana Velasquez Ochoa 3 , Angelo Vaccari 3 , Fabrizio Cavani 3 , Stefania Albonetti 3
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2016-07-07 00:00:00 , DOI: 10.1021/acs.jpcc.6b05428
Carlo Lucarelli 1, 2 , Simona Galli 1, 2 , Angelo Maspero 1 , Alessandro Cimino 1 , Claudia Bandinelli 3 , Alice Lolli 3 , Juliana Velasquez Ochoa 3 , Angelo Vaccari 3 , Fabrizio Cavani 3 , Stefania Albonetti 3
Affiliation
|
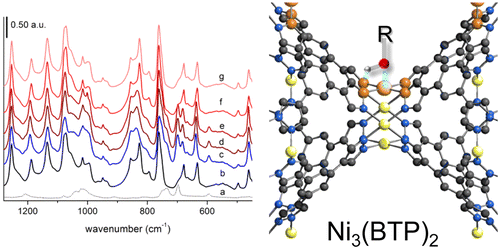
The three Ni-based metal–organic frameworks (MOFs) Ni(BDP), Ni(BPEB), and Ni3(BTP)2 [H2BDP = 1,4-(4-bispyrazolyl)benzene; H2BPEB = 1,4-bis(1H-pyrazol-4-ylethynyl)benzene; H3BTP = 1,3,5-tris(1H-pyrazol-4-yl)benzene], possessing square planar, potentially accessible metal sites, were preliminarily tested as catalysts in the base-free selective oxidation of hydroxymethylfurfural to 2,5-diformylfuran (DFF). While Ni(BDP) undergoes degradation, Ni3(BTP)2 is the most active of the three MOFs, yielding 27% DFF after 24 h with a selectivity close to 100% under relatively mild reaction conditions (120 °C, 30 bar O2, water as solvent). Upon flowing a model probe, in situ DRIFT and FT-IR spectroscopy were employed to rationalize the different performances of Ni(BPEB) and Ni3(BTP)2 in terms of adsorbate–adsorbent interactions: Not only hydrogen bonds are at work between the hydroxyl functionality of the probe and the pore walls of the MOF, but also and more importantly, bands ascribed to Ni-OR stretching are detected, denouncing the insurgence of Ni-probe interactions. The different intensity of these bands in the two cases confirms the different accessibility of the metal centers, as suggested by crystal structure analysis and catalytic tests.
中文翻译:

镍基MOF催化HMF氧化中的吸附剂-吸附剂相互作用:DRIFT和FT-IR洞察
三个基于镍的金属有机骨架(MOF)Ni(BDP),Ni(BPEB)和Ni 3(BTP)2 [H 2 BDP = 1,4-(4-双吡唑基)苯;H 2 BPEB = 1,4-双(1 H-吡唑-4-基乙炔基)苯; 初步测试了H 3 BTP = 1,3,5-三(1 H-吡唑-4-基)苯具有方形平面,可能易接近的金属位点,作为催化剂在羟甲基糠醛的无碱选择性氧化中反应成2, 5-二甲酰呋喃(DFF)。Ni(BDP)发生降解时,Ni 3(BTP)2是三种MOF中活性最高的,在相对温和的反应条件下(120°C,30 bar O),在24 h后产生的DFF为27%,选择性接近100%。2,以水为溶剂)。在流过模型探针后,采用原位DRIFT和FT-IR光谱法合理化了Ni(BPEB)和Ni 3(BTP)2在吸附物-吸附剂相互作用方面的不同性能:不仅氢键在两个键之间起作用检测到探针的羟基官能团和MOF的孔壁,而且更重要的是,检测到归因于Ni-OR拉伸的条带,这表明Ni-探针相互作用势在必行。如晶体结构分析和催化测试所示,在两种情况下,这些谱带的强度不同证实了金属中心的可及性不同。
更新日期:2016-07-07
中文翻译:

镍基MOF催化HMF氧化中的吸附剂-吸附剂相互作用:DRIFT和FT-IR洞察
三个基于镍的金属有机骨架(MOF)Ni(BDP),Ni(BPEB)和Ni 3(BTP)2 [H 2 BDP = 1,4-(4-双吡唑基)苯;H 2 BPEB = 1,4-双(1 H-吡唑-4-基乙炔基)苯; 初步测试了H 3 BTP = 1,3,5-三(1 H-吡唑-4-基)苯具有方形平面,可能易接近的金属位点,作为催化剂在羟甲基糠醛的无碱选择性氧化中反应成2, 5-二甲酰呋喃(DFF)。Ni(BDP)发生降解时,Ni 3(BTP)2是三种MOF中活性最高的,在相对温和的反应条件下(120°C,30 bar O),在24 h后产生的DFF为27%,选择性接近100%。2,以水为溶剂)。在流过模型探针后,采用原位DRIFT和FT-IR光谱法合理化了Ni(BPEB)和Ni 3(BTP)2在吸附物-吸附剂相互作用方面的不同性能:不仅氢键在两个键之间起作用检测到探针的羟基官能团和MOF的孔壁,而且更重要的是,检测到归因于Ni-OR拉伸的条带,这表明Ni-探针相互作用势在必行。如晶体结构分析和催化测试所示,在两种情况下,这些谱带的强度不同证实了金属中心的可及性不同。

































 京公网安备 11010802027423号
京公网安备 11010802027423号