Molecular Therapy ( IF 12.1 ) Pub Date : 2020-05-30 , DOI: 10.1016/j.ymthe.2020.05.021 Maria Caterina Rotiroti 1 , Chiara Buracchi 1 , Silvia Arcangeli 1 , Stefania Galimberti 2 , Maria Grazia Valsecchi 2 , Vincenzo Maria Perriello 3 , Tamas Rasko 4 , Gaia Alberti 1 , Chiara Francesca Magnani 1 , Claudia Cappuzzello 1 , Felix Lundberg 5 , Amit Pande 4 , Giuseppe Dastoli 1 , Martino Introna 6 , Marta Serafini 1 , Ettore Biagi 1 , Zsuzsanna Izsvák 4 , Andrea Biondi 1 , Sarah Tettamanti 1
|
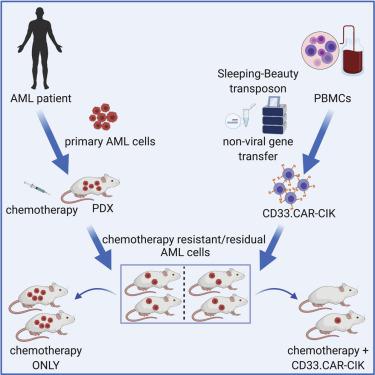
The successful implementation of chimeric antigen receptor (CAR)-T cell therapy in the clinical context of B cell malignancies has paved the way for further development in the more critical setting of acute myeloid leukemia (AML). Among the potentially targetable AML antigens, CD33 is insofar one of the main validated molecules. Here, we describe the feasibility of engineering cytokine-induced killer (CIK) cells with a CD33.CAR by using the latest optimized version of the non-viral Sleeping Beauty (SB) transposon system “SB100X-pT4.” This offers the advantage of improving CAR expression on CIK cells, while reducing the amount of DNA transposase as compared to the previously employed “SB11-pT” version. SB-modified CD33.CAR-CIK cells exhibited significant antileukemic activity in vitro and in vivo in patient-derived AML xenograft models, reducing AML development when administered as an “early treatment” and delaying AML progression in mice with established disease. Notably, by exploiting an already optimized xenograft chemotherapy model that mimics human induction therapy in mice, we demonstrated for the first time that CD33.CAR-CIK cells are also effective toward chemotherapy resistant/residual AML cells, further supporting its future clinical development and implementation within the current standard regimens.
中文翻译:

用改进的 SB 转座子系统修饰的 CAR-CIK 细胞靶向化学抗性 AML 患者衍生的异种移植物中的 CD33。
嵌合抗原受体 (CAR)-T 细胞疗法在 B 细胞恶性肿瘤的临床背景下的成功实施,为在更严重的急性髓性白血病 (AML) 环境中的进一步发展铺平了道路。在潜在的可靶向 AML 抗原中,CD33 是目前主要的经过验证的分子之一。在这里,我们描述了通过使用非病毒睡美人(SB) 转座子系统“SB100X-pT4”的最新优化版本,用 CD33.CAR 工程化细胞因子诱导的杀伤 (CIK) 细胞的可行性。与之前使用的“SB11-pT”版本相比,这提供了提高 CIK 细胞上 CAR 表达的优势,同时减少了 DNA 转座酶的数量。SB 修饰的 CD33.CAR-CIK 细胞在体外表现出显着的抗白血病活性和在体内在患者来源的AML异种移植模型中,当为“早期治疗”和延缓AML进展既定疾病的小鼠施用减少AML的发展。值得注意的是,通过利用已经优化的模拟小鼠人类诱导治疗的异种移植化疗模型,我们首次证明 CD33.CAR-CIK 细胞对化疗耐药/残留 AML 细胞也有效,进一步支持其未来的临床开发和实施在目前的标准方案中。































 京公网安备 11010802027423号
京公网安备 11010802027423号