Chemical Engineering Journal ( IF 13.3 ) Pub Date : 2020-05-30 , DOI: 10.1016/j.cej.2020.125570 Fu-Xiang Tian 1 , Wen-Kai Ye 1 , Bin Xu 2 , Xiao-Jun Hu 1 , Shi-Xu Ma 1 , Fan Lai 1 , Yu-Qiong Gao 3 , Hai-Bo Xing 1 , Wei-Hong Xia 1 , Bo Wang 1
|
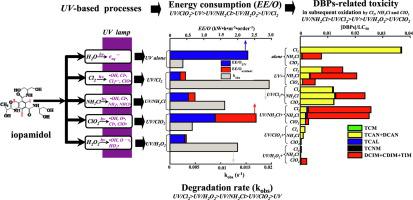
The UV-induced advanced oxidation processes (AOPs, including UV/Cl2, UV/NH2Cl, UV/ClO2 and UV/H2O2) degradation kinetics and energy requirements of iopamidol as well as DBPs-related toxicity in sequential disinfection were compared in this study. The photodegradation of iopamidol in these processes can be well described by pseudo-first-order model and the removal efficiency ranked in descending order of UV/Cl2 > UV/H2O2 > UV/NH2Cl > UV/ClO2 > UV. The synergistic effects could be attributed to diverse radical species generated in each system. Influencing factors of oxidant dosage, UV intensity, solution pH and water matrixes (Cl−, NH4+ and nature organic matter) were evaluated in detail. Higher oxidant dosages and greater UV intensities led to bigger pseudo-first-order rate constants (Kobs) in these processes, but the pH behaviors exhibited quite differently. The presence of Cl−, NH4+ and nature organic matter posed different effects on the degradation rate. The parameter of electrical energy per order (EE/O) was adopted to evaluate the energy requirements of the tested systems and it followed the trend of UV/ClO2 > UV > UV/NH2Cl > UV/H2O2 > UV/Cl2. Pretreatment of iopamidol by UV/Cl2 and UV/NH2Cl clearly enhanced the production of classical disinfection by-products (DBPs) and iodo-trihalomethanes (I-THMs) during subsequent oxidation while UV/ClO2 and UV/H2O2 exhibited almost elimination effect. From the perspective of weighted water toxicity, the risk ranking was UV/NH2Cl > UV/Cl2 > UV > UV/H2O2 > UV/ClO2. Among the discussed UV-driven AOPs, UV/Cl2 was proved to be the most cost-effective one for iopamidol removal while UV/ClO2 displayed overwhelming advantages in regulating the water toxicity associated with DBPs, especially I-THMs. The present results could provide some insights into the application of UV-activated AOPs technologies in tradeoffs between cost-effectiveness assessment and DBPs-related toxicity control of the disinfected waters containing iopamidol.
中文翻译:

紫外线诱导的 AOP(UV/Cl2、UV/NH2Cl、UV/ClO2 和 UV/H2O2)在碘帕醇降解中的比较:连续消毒过程中的动力学、能量需求和 DBP 相关毒性。
紫外线诱导的高级氧化过程(AOPs,包括UV/Cl 2、UV/NH 2 Cl、UV/ClO 2和UV/H 2 O 2)降解动力学和碘帕醇的能量需求以及 DBPs 相关毒性顺序本研究对消毒进行了比较。这些过程中碘帕醇的光降解可以用伪一级模型很好地描述,去除效率从高到低依次为UV/Cl 2 > UV/H 2 O 2 > UV/NH 2 Cl > UV/ClO 2 > 紫外线. 协同效应可归因于每个系统中产生的不同自由基物种。详细评价了氧化剂用量、紫外线强度、溶液pH值和水基质(Cl -、NH 4 +和天然有机物)的影响因素。在这些过程中,较高的氧化剂剂量和较大的紫外线强度会导致较大的准一级速率常数 (K obs ),但 pH 行为表现出截然不同的情况。Cl -、NH 4 +和天然有机物的存在对降解速率有不同的影响。单次电能参数(EE采用/ O ) 评估测试系统的能量需求,其趋势为UV/ClO 2 > UV > UV/NH 2 Cl > UV/H 2 O 2 > UV/Cl 2。用UV/Cl 2和UV/NH 2 Cl预处理碘帕醇明显增强了随后氧化过程中经典消毒副产物 (DBP) 和碘代三卤甲烷 (I-THM) 的产生,而UV/ClO 2和UV/H 2 O 2表现出几乎消除的效果。从加权水毒性来看,风险排序为UV/NH 2 Cl > UV/Cl 2 > UV > UV/H 2 O 2 > UV/ClO 2。在所讨论的紫外线驱动的AOP 中,UV/Cl 2被证明是去除碘帕醇最具成本效益的一种,而UV/ClO 2在调节与 DBP,尤其是 I-THM 相关的水毒性方面显示出压倒性优势。目前的结果可以为紫外线的应用提供一些见解- 激活 AOPs 技术在成本效益评估和含碘帕醇消毒水的 DBPs 相关毒性控制之间进行权衡。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号