当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Actinyl-Modified g-C3N4 as CO2 Activation Materials for Chemical Conversion and Environmental Remedy via an Artificial Photosynthetic Route.
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2020-05-29 , DOI: 10.1021/acs.inorgchem.0c00791 Raza Ullah Shah Bacha 1 , Li Li 1 , Yuan-Ru Guo 2 , Liqiang Jing 1 , Qing-Jiang Pan 1
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2020-05-29 , DOI: 10.1021/acs.inorgchem.0c00791 Raza Ullah Shah Bacha 1 , Li Li 1 , Yuan-Ru Guo 2 , Liqiang Jing 1 , Qing-Jiang Pan 1
Affiliation
|
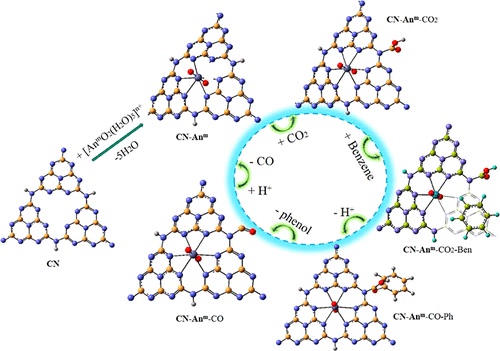
With the reported CO2 activation for the oxidation of benzene to phenol (-ENE → -OL) by the graphitic carbon nitride g-C3N4 (CN) via an artificial photosynthetic route as inspiration, high-valent actinyls (AnmO2)n+ (An = U, Np, Pu; m = VI, V; n = 2, 1) have been introduced for its further modification. Our calculations indicate thermodynamic spontaneity in the feasibility of g-C3N4-(AnmO2)n+ (CN-Anm) formation. The magnificent structural and electronic properties of CN-Anm are utilized for CO2 activation in terms of the rarely studied -ENE → -OL conversion. The calculated free energies show that most steps of the catalytic cycle are favored by CN-Anm complexes. The first step (carbamate formation) is slightly endothermic in all cases, where CN-U is 0.51 eV higher than CN and CN-Pu is −0.01 eV lower. All benzene addition reactions release energy, with that for CN-U being the lowest. The phenolate formation is favored by some actinyl complexes over CN, and CN-U is only 0.23 eV higher. The phenol release (resulting in formamide complexes) and CO desorption are exothermic for all CN-Anm. The overall process suggests the improved catalytic performance of actinyl-modified CN materials, and the slightly depleted uranyl-carbon nitride could be one of the promising catalysts.
中文翻译:

in系修饰的g-C3N4作为CO2活化材料,可通过人工光合作用途径进行化学转化和环境修复。
据报道,通过人工光合作用的石墨碳氮化物gC 3 N 4(CN)激发了CO 2活化苯氧化为苯酚(-ENE→-OL)的作用,因此,高价act化基(An m O 2)n +(An = U,Np,Pu; m = VI,V; n = 2,1)已被引入以作进一步修改。我们的计算表明在gC 3 N 4-(An m O 2)n +(CN-An m)形成。就很少研究的-ENE→-OL转化而言,CN-An m的出色的结构和电子性质可用于CO 2活化。计算出的自由能表明,CN-An m配合物有利于催化循环的大多数步骤。在所有情况下,第一步(氨基甲酸酯形成)都略有吸热,其中CN-U比CN高0.51 eV ,CN-Pu低-0.01 eV。所有苯加成反应均释放能量,其中CN-U的能量最低。某些act基配合物比CN和CN-U更有利于酚盐的形成仅高出0.23 eV。所有CN-An m的苯酚释放(导致甲酰胺络合物)和CO解吸是放热的。整个过程表明,in化基改性的CN材料具有更高的催化性能,略微耗尽的铀酰-碳氮化物可能是有前途的催化剂之一。
更新日期:2020-05-29
中文翻译:

in系修饰的g-C3N4作为CO2活化材料,可通过人工光合作用途径进行化学转化和环境修复。
据报道,通过人工光合作用的石墨碳氮化物gC 3 N 4(CN)激发了CO 2活化苯氧化为苯酚(-ENE→-OL)的作用,因此,高价act化基(An m O 2)n +(An = U,Np,Pu; m = VI,V; n = 2,1)已被引入以作进一步修改。我们的计算表明在gC 3 N 4-(An m O 2)n +(CN-An m)形成。就很少研究的-ENE→-OL转化而言,CN-An m的出色的结构和电子性质可用于CO 2活化。计算出的自由能表明,CN-An m配合物有利于催化循环的大多数步骤。在所有情况下,第一步(氨基甲酸酯形成)都略有吸热,其中CN-U比CN高0.51 eV ,CN-Pu低-0.01 eV。所有苯加成反应均释放能量,其中CN-U的能量最低。某些act基配合物比CN和CN-U更有利于酚盐的形成仅高出0.23 eV。所有CN-An m的苯酚释放(导致甲酰胺络合物)和CO解吸是放热的。整个过程表明,in化基改性的CN材料具有更高的催化性能,略微耗尽的铀酰-碳氮化物可能是有前途的催化剂之一。

































 京公网安备 11010802027423号
京公网安备 11010802027423号