当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
NMR Spectroscopic Studies Reveal the Critical Role of the Isopeptide Bond in Forming the Otherwise Unstable SpyTag-SpyCatcher Mutant Complexes.
Biochemistry ( IF 2.9 ) Pub Date : 2020-05-29 , DOI: 10.1021/acs.biochem.0c00287 Nan Zhang 1, 2 , Jing Liu 1, 3 , Yajie Liu 1, 2 , Wen-Hao Wu 1, 2 , Jing Fang 1, 2 , Xiao-Di Da 1, 2 , Shenlin Wang 1, 3 , Wen-Bin Zhang 1, 2
Biochemistry ( IF 2.9 ) Pub Date : 2020-05-29 , DOI: 10.1021/acs.biochem.0c00287 Nan Zhang 1, 2 , Jing Liu 1, 3 , Yajie Liu 1, 2 , Wen-Hao Wu 1, 2 , Jing Fang 1, 2 , Xiao-Di Da 1, 2 , Shenlin Wang 1, 3 , Wen-Bin Zhang 1, 2
Affiliation
|
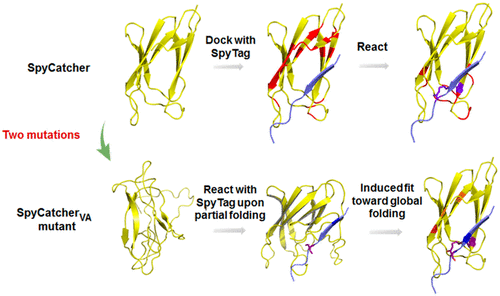
The interplay between protein folding and chemical reaction has been an intriguing subject. In this contribution, we report the study of SpyTag and SpyCatcher reactive mutants using a combination of sodium dodecyl sulfate–polyacrylamide gel electrophoresis, liquid chromatography and mass spectrometry, circular dichroism, and NMR spectroscopy. It was found that the wild-type SpyCatcher is well-folded in solution and docks with SpyTag to form an intermediate that promotes isopeptide bond formation. By contrast, the double mutant SpyCatcherVA is disordered in solution yet remains reactive toward SpyTag, forming a well-folded covalent complex. Control experiments using the catalytically inactive mutants further reveal the critical role of the isopeptide bond in stabilizing the otherwise loose SpyTag–SpyCatcherVA complex, amplifying the effect of the minute sequence disparity. We believe that the synergy between protein folding and isopeptide bonding is an effective way to enhance protein stability and engineer protein–protein interactions.
中文翻译:

NMR光谱研究揭示了异肽键在形成否则不稳定的SpyTag-SpyCatcher突变体复合物中的关键作用。
蛋白质折叠和化学反应之间的相互作用一直是一个有趣的话题。在这项贡献中,我们报告了十二烷基硫酸钠-聚丙烯酰胺凝胶电泳,液相色谱和质谱,圆二色性和NMR光谱相结合的SpyTag和SpyCatcher反应性突变体的研究。发现野生型SpyCatcher在溶液中折叠良好,并与SpyTag对接以形成促进异肽键形成的中间体。相比之下,双重突变体SpyCatcher VA在溶液中无序,但仍对SpyTag具有反应性,形成折叠良好的共价复合物。使用无催化活性的突变体的对照实验进一步揭示了异肽键在稳定原本松散的SpyTag–SpyCatcher中的关键作用VA复合物,可放大微小序列差异的影响。我们认为,蛋白质折叠和异肽键合之间的协同作用是增强蛋白质稳定性和工程化蛋白质与蛋白质相互作用的有效方法。
更新日期:2020-06-23
中文翻译:

NMR光谱研究揭示了异肽键在形成否则不稳定的SpyTag-SpyCatcher突变体复合物中的关键作用。
蛋白质折叠和化学反应之间的相互作用一直是一个有趣的话题。在这项贡献中,我们报告了十二烷基硫酸钠-聚丙烯酰胺凝胶电泳,液相色谱和质谱,圆二色性和NMR光谱相结合的SpyTag和SpyCatcher反应性突变体的研究。发现野生型SpyCatcher在溶液中折叠良好,并与SpyTag对接以形成促进异肽键形成的中间体。相比之下,双重突变体SpyCatcher VA在溶液中无序,但仍对SpyTag具有反应性,形成折叠良好的共价复合物。使用无催化活性的突变体的对照实验进一步揭示了异肽键在稳定原本松散的SpyTag–SpyCatcher中的关键作用VA复合物,可放大微小序列差异的影响。我们认为,蛋白质折叠和异肽键合之间的协同作用是增强蛋白质稳定性和工程化蛋白质与蛋白质相互作用的有效方法。

































 京公网安备 11010802027423号
京公网安备 11010802027423号