当前位置:
X-MOL 学术
›
ACS Appl. Energy Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Nanostructured CoS2-Decorated Hollow Carbon Spheres: A Performance Booster for Li-Ion/Sulfur Batteries
ACS Applied Energy Materials ( IF 5.4 ) Pub Date : 2020-05-27 , DOI: 10.1021/acsaem.0c00699 Jicheng Jiang 1 , Qining Fan 1 , Zhi Zheng 1 , Mohammad Rejaul Kaiser 1, 2 , Qinfen Gu 3 , Shulei Chou 1 , Konstantin Konstantinov 1 , Jiazhao Wang 1
ACS Applied Energy Materials ( IF 5.4 ) Pub Date : 2020-05-27 , DOI: 10.1021/acsaem.0c00699 Jicheng Jiang 1 , Qining Fan 1 , Zhi Zheng 1 , Mohammad Rejaul Kaiser 1, 2 , Qinfen Gu 3 , Shulei Chou 1 , Konstantin Konstantinov 1 , Jiazhao Wang 1
Affiliation
|
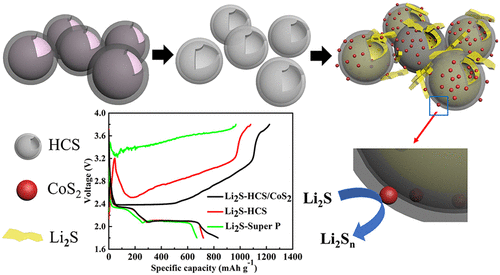
The practical application of Li–S batteries is being obstructed by severe safety concerns due to lithium dendrites. The Li-ion/sulfur batteries, which use Li2S as the cathode and can be coupled with different anodes (e.g., Si or Sn), can eliminate the safety issues related to lithium metal. To improve the performance of Li2S cathode for Li-ion/sulfur batteries, CoS2-decorated hollow carbon spheres (HCS) are first explored as a conductive matrix for the Li2S cathode. Hollow carbon spheres (HCS) possesses a strong tendency to physically absorb and trap high-order lithium polysulfides, while the CoS2 can chemically bond with low-order lithium polysulfides. Moreover, CoS2 has a catalytic effect that can reduce the energy barrier in the first charge. In situ synchrotron X-ray diffraction has clarified the catalytic mechanism of CoS2 toward barrier reduction. CoS2 can boost the electrochemical reactions from Li2S to polysulfide and act as a redox mediator, lowering the overpotential of Li2S in the first charge process, resulting in less electrolyte decomposition, stable cycling performance, and higher capacity. The data show that CoS2-decorated hollow carbon spheres have a higher initial specific capacity and better capacity retention, with specific capacity of 831 mA h g–1 and capacity retention of 79.5% after 100 cycles, which is much better than the performance of hollow carbon spheres (Li2S–HCS) alone. The full cell core–shell Si@C||Li2S–HCS/CoS2 shows a specific capacity of 650 mA h g–1 and a capacity retention of 65% after 50 cycles at an average voltage of 1.6 V with low electrolyte to sulfur (E/S) and anode to cathode (A/C) ratio.
中文翻译:

纳米结构的CoS 2装饰空心碳球:锂离子/硫电池的性能增强器
由于锂枝晶引起的严重安全隐患,阻碍了Li-S电池的实际应用。使用Li 2 S作为阴极并可以与不同的阳极(例如,Si或Sn)耦合的锂离子/硫电池可以消除与锂金属有关的安全问题。为了改善用于锂离子/硫电池的Li 2 S阴极的性能,首先探索了装饰有CoS 2的空心碳球(HCS)作为Li 2 S阴极的导电基质。空心碳球(HCS)具有强烈的物理吸收和捕获高阶多硫化锂的趋势,而CoS 2可以与低阶多硫化锂化学键合。此外,CoS 2具有催化作用,可以减少第一次充电时的能垒。原位同步加速器X射线衍射已经阐明了CoS 2朝向势垒还原的催化机理。CoS 2可以促进从Li 2 S到多硫化物的电化学反应,并充当氧化还原介体,降低第一次充电过程中Li 2 S的过电势,从而减少电解质分解,稳定的循环性能和更高的容量。数据表明,用CoS 2装饰的空心碳球具有更高的初始比容量和更好的容量保持率,比容量为831 mA hg –1100次循环后的容量保持率为79.5%,这比单独使用空心碳球(Li 2 S–HCS)的性能要好得多。全电池核-壳Si @ C || Li 2 S–HCS / CoS 2在1.6 V的平均电压下,经过50次循环,在低电解液下,显示了650 mA hg –1的比容量和65%的容量保持率。硫(E / S)和阳极对阴极(A / C)的比率。
更新日期:2020-05-27
中文翻译:

纳米结构的CoS 2装饰空心碳球:锂离子/硫电池的性能增强器
由于锂枝晶引起的严重安全隐患,阻碍了Li-S电池的实际应用。使用Li 2 S作为阴极并可以与不同的阳极(例如,Si或Sn)耦合的锂离子/硫电池可以消除与锂金属有关的安全问题。为了改善用于锂离子/硫电池的Li 2 S阴极的性能,首先探索了装饰有CoS 2的空心碳球(HCS)作为Li 2 S阴极的导电基质。空心碳球(HCS)具有强烈的物理吸收和捕获高阶多硫化锂的趋势,而CoS 2可以与低阶多硫化锂化学键合。此外,CoS 2具有催化作用,可以减少第一次充电时的能垒。原位同步加速器X射线衍射已经阐明了CoS 2朝向势垒还原的催化机理。CoS 2可以促进从Li 2 S到多硫化物的电化学反应,并充当氧化还原介体,降低第一次充电过程中Li 2 S的过电势,从而减少电解质分解,稳定的循环性能和更高的容量。数据表明,用CoS 2装饰的空心碳球具有更高的初始比容量和更好的容量保持率,比容量为831 mA hg –1100次循环后的容量保持率为79.5%,这比单独使用空心碳球(Li 2 S–HCS)的性能要好得多。全电池核-壳Si @ C || Li 2 S–HCS / CoS 2在1.6 V的平均电压下,经过50次循环,在低电解液下,显示了650 mA hg –1的比容量和65%的容量保持率。硫(E / S)和阳极对阴极(A / C)的比率。

































 京公网安备 11010802027423号
京公网安备 11010802027423号