当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Transformations of Aryl Ketones via Ligand-Promoted C-C Bond Activation.
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-05-28 , DOI: 10.1002/anie.202006740 Hanyuan Li 1 , Biao Ma 1 , Qi-Sheng Liu 1 , Mei-Ling Wang 2 , Zhen-Yu Wang 3 , Hui Xu 1 , Ling-Jun Li 1 , Xing Wang 1 , Hui-Xiong Dai 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-05-28 , DOI: 10.1002/anie.202006740 Hanyuan Li 1 , Biao Ma 1 , Qi-Sheng Liu 1 , Mei-Ling Wang 2 , Zhen-Yu Wang 3 , Hui Xu 1 , Ling-Jun Li 1 , Xing Wang 1 , Hui-Xiong Dai 1
Affiliation
|
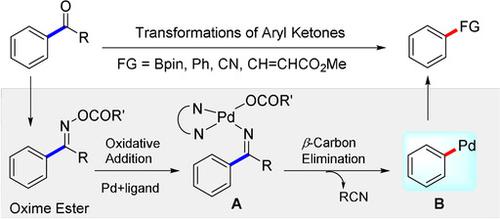
The coupling of aromatic electrophiles (aryl halides, aryl ethers, aryl acids, aryl nitriles etc.) with nucleophiles is a core methodology for the synthesis of aryl compounds. Transformations of aryl ketones in an analogous manner via carbon–carbon bond activation could greatly expand the toolbox for the synthesis of aryl compounds due to the abundance of aryl ketones. An exploratory study of this approach is typically based on carbon–carbon cleavage triggered by ring‐strain release and chelation assistance, and the products are also limited to a specific structural motif. Here we report a ligand‐promoted β‐carbon elimination strategy to activate the carbon–carbon bonds, which results in a range of transformations of aryl ketones, leading to useful aryl borates, and also to biaryls, aryl nitriles, and aryl alkenes. The use of a pyridine‐oxazoline ligand is crucial for this catalytic transformation. A gram‐scale borylation reaction of an aryl ketone via a simple one‐pot operation is reported. The potential utility of this strategy is also demonstrated by the late‐stage diversification of drug molecules probenecid, adapalene, and desoxyestrone, the fragrance tonalid as well as the natural product apocynin.
中文翻译:

通过配体促进的CC键活化来转化芳基酮。
芳族亲电试剂(芳基卤化物,芳基醚,芳基酸,芳基腈等)与亲核试剂的偶联是合成芳基化合物的核心方法。由于芳基酮的丰度,通过碳-碳键活化以类似方式进行芳基酮的转化可大大扩展合成芳基化合物的工具箱。这种方法的探索性研究通常基于环应变释放和螯合辅助引发的碳-碳裂解,并且产物也仅限于特定的结构基序。在这里,我们报告了一种配体促进β-碳消除的策略,以激活碳-碳键,这导致芳基酮发生一系列转化,从而生成有用的芳基硼酸酯,以及联芳基,芳基腈和芳基烯烃。吡啶-恶唑啉配体的使用对于这种催化转化至关重要。据报道,通过简单的一锅操作,芳基酮的克级硼酸酯化反应。该策略的潜在效用还通过丙磺舒,阿达帕林和脱氧雌酮,芬芳类香囊素和载脂蛋白的天然产物的晚期药物分子多样化而得到证明。
更新日期:2020-05-28
中文翻译:

通过配体促进的CC键活化来转化芳基酮。
芳族亲电试剂(芳基卤化物,芳基醚,芳基酸,芳基腈等)与亲核试剂的偶联是合成芳基化合物的核心方法。由于芳基酮的丰度,通过碳-碳键活化以类似方式进行芳基酮的转化可大大扩展合成芳基化合物的工具箱。这种方法的探索性研究通常基于环应变释放和螯合辅助引发的碳-碳裂解,并且产物也仅限于特定的结构基序。在这里,我们报告了一种配体促进β-碳消除的策略,以激活碳-碳键,这导致芳基酮发生一系列转化,从而生成有用的芳基硼酸酯,以及联芳基,芳基腈和芳基烯烃。吡啶-恶唑啉配体的使用对于这种催化转化至关重要。据报道,通过简单的一锅操作,芳基酮的克级硼酸酯化反应。该策略的潜在效用还通过丙磺舒,阿达帕林和脱氧雌酮,芬芳类香囊素和载脂蛋白的天然产物的晚期药物分子多样化而得到证明。































 京公网安备 11010802027423号
京公网安备 11010802027423号