Chem ( IF 19.1 ) Pub Date : 2020-05-28 , DOI: 10.1016/j.chempr.2020.04.022 Penghao Jia , Qingyao Li , Wei Chuen Poh , Heming Jiang , Haiwang Liu , Hongping Deng , Jie Wu
|
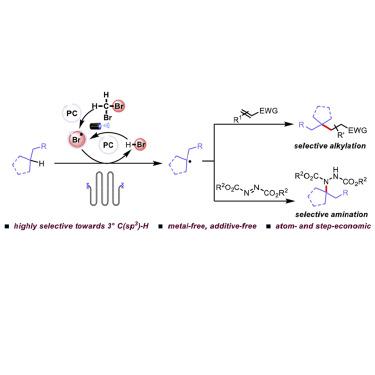
Substantial effort is currently being devoted to selective C(sp3)–H bond functionalization, and photocatalysis has presented enormous potential opportunities in this research field. Here, we developed a visible-light-induced functionalization of unactivated C(sp3)–H bonds via the synergistic effects of an organo-photoredox catalyst and a bromine-based hydrogen atom transfer agent. By applying CH2Br2 as both the solvent and the bromine radical source, the alkylation and amination of tertiary C(sp3)–H bonds were achieved in a highly selective fashion. This study represents the first example of selective activation of unactivated alkanes by bromine radicals in a catalytic and metal-free manner. Good reactivity was achieved by using a sealed microtubing reactor or by adding a proper amount of water. This highly selective C(sp3)–H functionalization protocol offers a new paradigm for the direct synthesis of valuable compounds from abundant alkane feedstocks in a convenient and green manner.
中文翻译:

光促进的溴-自由基介导的选择性烷基化和未活化的C(sp 3)-H键的胺化
目前正致力于选择性C(sp 3)-H键的功能化,光催化已在该研究领域提供了巨大的潜在机会。在这里,我们通过有机光氧化还原催化剂和溴基氢原子转移剂的协同作用,开发了可见光诱导的未激活的C(sp 3)–H键的官能化。通过同时使用CH 2 Br 2作为溶剂和溴自由基源,可将叔C(sp 3)– H键以高度选择性的方式实现。这项研究代表了溴自由基以无金属催化方式选择性活化未活化烷烃的第一个实例。通过使用密封的微管反应器或添加适量的水可获得良好的反应性。这种高度选择性的C(sp 3)–H功能化方案为以便捷,绿色的方式从丰富的烷烃原料直接合成有价值的化合物提供了新的范例。






























 京公网安备 11010802027423号
京公网安备 11010802027423号