当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mechanism of Color and Photoacidity Tuning for the Protonated Green Fluorescent Protein Chromophore
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-05-26 , DOI: 10.1021/jacs.0c02796 Chi-Yun Lin 1 , Steven G Boxer 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-05-26 , DOI: 10.1021/jacs.0c02796 Chi-Yun Lin 1 , Steven G Boxer 1
Affiliation
|
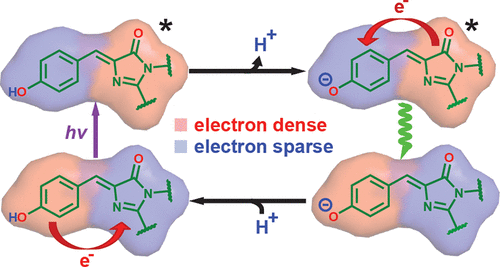
The neutral or A state of the green fluorescent protein (GFP) chromophore is a remarkable example of a photoacid naturally embedded in the protein environment and accounts for the large Stokes shift of GFP in response to near UV excitation. Its color tuning mechanism has been largely overlooked, as it is less preferred for imaging applications than the redder anionic or B state. Past studies, based on site-directed mutagenesis or solvatochromism of the isolated chromophore, have concluded that its color tuning range is much narrower than its anionic counterpart. However, as we performed extensive investigation on more GFP mutants, we found that the color of the neutral chromophore can be more sensitive to protein electrostatics than the anionic counterpart. Electronic Stark spectroscopy reveals a fundamentally different electrostatic color tuning mechanism for the neutral state of the chromophore that demands a three-form model compared with that of the anionic state, which requires only two forms. Specifically, an underlying zwitterionic charge transfer state is required to explain its sensitivity to electrostatics. As the Stokes shift is tightly linked to excited-state proton transfer (ESPT) of the protonated chromophore, we infer design principles of the GFP chromophore as a photoacid through the color tuning mechanisms of both protonation states. The three-form model could also be applied to similar biological and nonbiological dyes and complements the failure of two-form model for donor-acceptor systems with localized ground-state electronic distributions.
中文翻译:

质子化绿色荧光蛋白发色团的颜色和光酸调节机制
绿色荧光蛋白 (GFP) 发色团的中性或 A 状态是自然嵌入蛋白质环境中的光酸的一个显着例子,并解释了 GFP 响应近紫外线激发的大斯托克斯位移。它的颜色调节机制在很大程度上被忽视了,因为与较红的阴离子或 B 状态相比,它不太适合成像应用。过去的研究基于分离生色团的定点诱变或溶剂化变色,得出的结论是其颜色调节范围比其阴离子对应物窄得多。然而,当我们对更多 GFP 突变体进行广泛研究时,我们发现中性发色团的颜色对蛋白质静电比阴离子对应物更敏感。电子斯塔克光谱揭示了生色团中性状态的一种根本不同的静电颜色调谐机制,与阴离子状态相比,生色团需要一种三形式模型,而阴离子状态只需要两种形式。具体来说,需要潜在的两性离子电荷转移状态来解释其对静电的敏感性。由于斯托克斯位移与质子化发色团的激发态质子转移 (ESPT) 密切相关,我们通过两种质子化状态的颜色调节机制推断出 GFP 发色团作为光酸的设计原理。三形式模型也可以应用于类似的生物和非生物染料,并补充了具有局部基态电子分布的供体 - 受体系统的二形式模型的失败。
更新日期:2020-05-26
中文翻译:

质子化绿色荧光蛋白发色团的颜色和光酸调节机制
绿色荧光蛋白 (GFP) 发色团的中性或 A 状态是自然嵌入蛋白质环境中的光酸的一个显着例子,并解释了 GFP 响应近紫外线激发的大斯托克斯位移。它的颜色调节机制在很大程度上被忽视了,因为与较红的阴离子或 B 状态相比,它不太适合成像应用。过去的研究基于分离生色团的定点诱变或溶剂化变色,得出的结论是其颜色调节范围比其阴离子对应物窄得多。然而,当我们对更多 GFP 突变体进行广泛研究时,我们发现中性发色团的颜色对蛋白质静电比阴离子对应物更敏感。电子斯塔克光谱揭示了生色团中性状态的一种根本不同的静电颜色调谐机制,与阴离子状态相比,生色团需要一种三形式模型,而阴离子状态只需要两种形式。具体来说,需要潜在的两性离子电荷转移状态来解释其对静电的敏感性。由于斯托克斯位移与质子化发色团的激发态质子转移 (ESPT) 密切相关,我们通过两种质子化状态的颜色调节机制推断出 GFP 发色团作为光酸的设计原理。三形式模型也可以应用于类似的生物和非生物染料,并补充了具有局部基态电子分布的供体 - 受体系统的二形式模型的失败。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号