当前位置:
X-MOL 学术
›
ACS Appl. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ultrafast Growth of a Cu(OH)2-CuO Nanoneedle Array on Cu Foil for Methanol Oxidation Electrocatalysis.
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2020-05-27 , DOI: 10.1021/acsami.0c08979 Sengeni Anantharaj 1 , Hisashi Sugime 1 , Suguru Noda 1, 2
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2020-05-27 , DOI: 10.1021/acsami.0c08979 Sengeni Anantharaj 1 , Hisashi Sugime 1 , Suguru Noda 1, 2
Affiliation
|
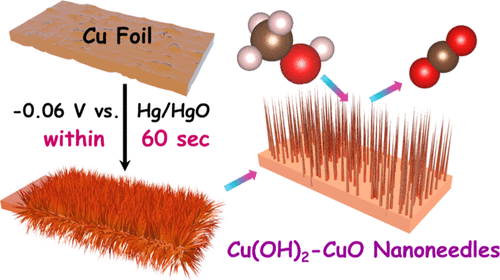
A swift potentiostatic anodization method for growing a 5–7 μm tall nanoneedle array of Cu(OH)2–CuO on Cu foil within 100 s has been developed. This catalytic electrode when screened for methanol oxidation electrocatalysis in 1 M KOH with 0.5 M methanol, delivered a current density as high as 70 ± 10 mA cm–2 at 0.65 V versus Hg/HgO which is superior to the performance of many related catalysts reported earlier. The observed activity enhancement is attributed to the formation of both Cu(OH)2–CuO nanoneedle arrays of high active surface area over the metallic Cu foil. In addition, the Cu(OH)2–CuO/Cu electrode had also exhibited excellent stability upon prolonged potentiostatic electrocatalytic oxidation of methanol while retaining the charge-transfer characteristics. Growth of such highly ordered assembly of Cu(OH)2–CuO nanoneedles within a minute has never been achieved before. When compared to its oxygen evolution reaction activity, the addition of 0.5 M methanol has lowered the overpotential at 10 mA cm–2 by 334 mV, which is significant. This encourages the use of methanol as a sacrificial anolyte for energy-saving production of H2 from water electrolysis.
中文翻译:

Cu(OH)2-CuO纳米针阵列在铜箔上的超快生长,用于甲醇氧化电催化。
已经开发了一种快速恒电位阳极氧化方法,可在100 s内在Cu箔上生长5–7μm高的Cu(OH)2 -CuO纳米针阵列。当在0.5 M甲醇中在1 M KOH中用0.5 M甲醇对甲醇氧化电催化进行筛选时,该催化电极在0.65 V电压下相对于Hg / HgO的电流密度高达70±10 mA cm –2,优于许多报道的相关催化剂的性能。较早。观察到的活性增强归因于在金属铜箔上具有高活性表面积的两个Cu(OH)2 -CuO纳米针阵列的形成。另外,Cu(OH)2-CuO / Cu电极在长时间的甲醇恒电位电催化氧化反应中也表现出出色的稳定性,同时保持了电荷转移特性。这种高度有序的Cu(OH)2 -CuO纳米针组装在一分钟之内从未实现。与它的析氧反应活性相比,添加0.5 M甲醇可将10 mA cm –2处的过电势降低334 mV,这一点非常重要。这鼓励使用甲醇作为牺牲阳极电解液,以通过水电解节能地生产H 2。
更新日期:2020-05-27
中文翻译:

Cu(OH)2-CuO纳米针阵列在铜箔上的超快生长,用于甲醇氧化电催化。
已经开发了一种快速恒电位阳极氧化方法,可在100 s内在Cu箔上生长5–7μm高的Cu(OH)2 -CuO纳米针阵列。当在0.5 M甲醇中在1 M KOH中用0.5 M甲醇对甲醇氧化电催化进行筛选时,该催化电极在0.65 V电压下相对于Hg / HgO的电流密度高达70±10 mA cm –2,优于许多报道的相关催化剂的性能。较早。观察到的活性增强归因于在金属铜箔上具有高活性表面积的两个Cu(OH)2 -CuO纳米针阵列的形成。另外,Cu(OH)2-CuO / Cu电极在长时间的甲醇恒电位电催化氧化反应中也表现出出色的稳定性,同时保持了电荷转移特性。这种高度有序的Cu(OH)2 -CuO纳米针组装在一分钟之内从未实现。与它的析氧反应活性相比,添加0.5 M甲醇可将10 mA cm –2处的过电势降低334 mV,这一点非常重要。这鼓励使用甲醇作为牺牲阳极电解液,以通过水电解节能地生产H 2。

































 京公网安备 11010802027423号
京公网安备 11010802027423号