当前位置:
X-MOL 学术
›
Colloids Surf. A Physicochem. Eng. Aspects
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
New insights into the removal of antimony from water using an iron-based metal-organic framework: adsorption behaviors and mechanisms
Colloids and Surfaces A: Physicochemical and Engineering Aspects ( IF 4.9 ) Pub Date : 2020-10-01 , DOI: 10.1016/j.colsurfa.2020.125054 Kuan Cheng , Yi-nan Wu , Bingru Zhang , Fengting Li
Colloids and Surfaces A: Physicochemical and Engineering Aspects ( IF 4.9 ) Pub Date : 2020-10-01 , DOI: 10.1016/j.colsurfa.2020.125054 Kuan Cheng , Yi-nan Wu , Bingru Zhang , Fengting Li
|
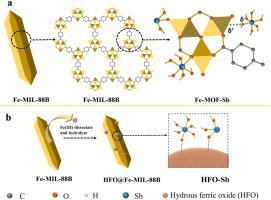
Abstract Increasing antimony (Sb) pollution has posed severe health threats to human beings and the environment. In the study, iron-based metal-organic framework Fe-MIL-88B was synthesized at low temperature (40 °C) via ethanol as solvent. And the removal of Sb(III) and Sb(V) by using Fe-MIL-88B were investigated systematically with the effects of pH, adsorbent dosages, adsorption time, initial concentration and co-existing anions. Rapid adsorption kinetics and high adsorption capacities g− g− were obtained. Sb was also removed by Fe-MIL-88B from natural water successfully. And the concentrations of residual Sb(III) and Sb(V) were below 5 μg L−1, meeting the drinking water standard. New insights into the possible removal mechanisms were provided that the coordination between Fe3-μ3-oxo clusters of Fe-MIL-88B and Sb attributed to the adsorption of Sb. And more active adsorption sites were generated by in-situ formed hydrous ferric oxide (HFO) on the surface of the adsorbent, which also improved the removal of Sb. And the mechanisms were supported by X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FTIR) and X-ray photoelectron spectroscopy (XPS) characterizations. The results revealed that Fe-MIL-88B is a promising and efficient adsorbent for Sb removal in practical applications.
中文翻译:

使用铁基金属有机骨架从水中去除锑的新见解:吸附行为和机制
摘要 日益严重的锑(Sb)污染对人类和环境造成了严重的健康威胁。在该研究中,以乙醇为溶剂,在低温(40°C)下合成了铁基金属有机骨架 Fe-MIL-88B。并且系统地研究了使用 Fe-MIL-88B 去除 Sb(III) 和 Sb(V) 的 pH、吸附剂剂量、吸附时间、初始浓度和共存阴离子的影响。获得了快速的吸附动力学和高吸附容量 g-g-。Fe-MIL-88B 也成功地从天然水中去除了 Sb。且残留Sb(III)和Sb(V)浓度均低于5 μg L-1,符合饮用水标准。提供了对可能的去除机制的新见解,即 Fe-MIL-88B 和 Sb 的 Fe3-μ3-oxo 簇之间的配位归因于 Sb 的吸附。并且在吸附剂表面原位形成的水合氧化铁(HFO)产生了更多的活性吸附位点,这也提高了Sb的去除率。X 射线衍射 (XRD)、傅里叶变换红外光谱 (FTIR) 和 X 射线光电子能谱 (XPS) 表征支持了该机制。结果表明,Fe-MIL-88B 是一种在实际应用中去除 Sb 的有前途且有效的吸附剂。傅里叶变换红外光谱 (FTIR) 和 X 射线光电子能谱 (XPS) 表征。结果表明,Fe-MIL-88B 是一种在实际应用中去除 Sb 的有前途且有效的吸附剂。傅里叶变换红外光谱 (FTIR) 和 X 射线光电子能谱 (XPS) 表征。结果表明,Fe-MIL-88B 是一种在实际应用中去除 Sb 的有前途且有效的吸附剂。
更新日期:2020-10-01
中文翻译:

使用铁基金属有机骨架从水中去除锑的新见解:吸附行为和机制
摘要 日益严重的锑(Sb)污染对人类和环境造成了严重的健康威胁。在该研究中,以乙醇为溶剂,在低温(40°C)下合成了铁基金属有机骨架 Fe-MIL-88B。并且系统地研究了使用 Fe-MIL-88B 去除 Sb(III) 和 Sb(V) 的 pH、吸附剂剂量、吸附时间、初始浓度和共存阴离子的影响。获得了快速的吸附动力学和高吸附容量 g-g-。Fe-MIL-88B 也成功地从天然水中去除了 Sb。且残留Sb(III)和Sb(V)浓度均低于5 μg L-1,符合饮用水标准。提供了对可能的去除机制的新见解,即 Fe-MIL-88B 和 Sb 的 Fe3-μ3-oxo 簇之间的配位归因于 Sb 的吸附。并且在吸附剂表面原位形成的水合氧化铁(HFO)产生了更多的活性吸附位点,这也提高了Sb的去除率。X 射线衍射 (XRD)、傅里叶变换红外光谱 (FTIR) 和 X 射线光电子能谱 (XPS) 表征支持了该机制。结果表明,Fe-MIL-88B 是一种在实际应用中去除 Sb 的有前途且有效的吸附剂。傅里叶变换红外光谱 (FTIR) 和 X 射线光电子能谱 (XPS) 表征。结果表明,Fe-MIL-88B 是一种在实际应用中去除 Sb 的有前途且有效的吸附剂。傅里叶变换红外光谱 (FTIR) 和 X 射线光电子能谱 (XPS) 表征。结果表明,Fe-MIL-88B 是一种在实际应用中去除 Sb 的有前途且有效的吸附剂。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号