当前位置:
X-MOL 学术
›
ACS Chem. Neurosci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Green Tea Extracts EGCG and EGC Display Distinct Mechanisms in Disrupting Aβ42 Protofibril.
ACS Chemical Neuroscience ( IF 4.1 ) Pub Date : 2020-05-22 , DOI: 10.1021/acschemneuro.0c00277
Chendi Zhan 1 , Yujie Chen 1 , Yiming Tang 1 , Guanghong Wei 1
ACS Chemical Neuroscience ( IF 4.1 ) Pub Date : 2020-05-22 , DOI: 10.1021/acschemneuro.0c00277
Chendi Zhan 1 , Yujie Chen 1 , Yiming Tang 1 , Guanghong Wei 1
Affiliation
|
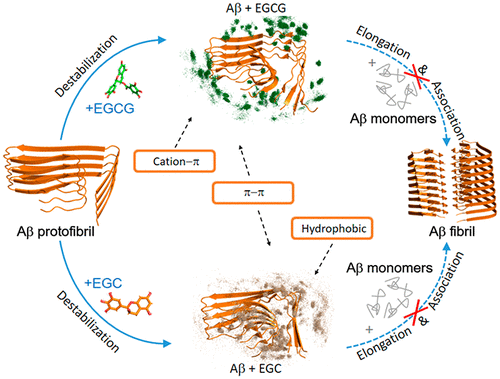
The amyloid beta (Aβ) fibrillar aggregate is the hallmark of Alzheimer’s disease (AD). Disassembling preformed fibril or inhibiting Aβ aggregation is considered as a therapeutic strategy for AD. Increasing evidence shows that green tea extracts, epigallocatechin-3-gallate (EGCG, containing an extra gallic acid ester group compared to EGC) and epigallocatechin (EGC), can disassociate Aβ fibrils and attenuate Aβ toxicity. However, the underlying molecular mechanism is poorly understood. Herein, we performed microsecond all-atom molecular dynamics (MD) simulations to investigate the influences of EGCG/EGC on the newly cryo-EM resolved LS-shaped Aβ42 protofibrils and their detailed interactions. MD simulations demonstrate that both EGCG and EGC can disrupt Aβ42 protofibril and EGCG displays a higher disruptive capacity than EGC. EGCG alters the L-shape of Aβ42 protofibril by breaking the hydrogen bond between H6 and E11 through π–π interactions with residues H14/Y10 and hydrogen-bonding interactions with E11, while EGC remodels the L-shape by inserting into the hydrophobic core formed by A2, F4, L34, and V36 and via aromatics interaction with H6/Y10. EGCG disrupts the salt bridges between the K28 side chain and A42 COO– through hydrogen-bonding interaction with A42 and cation−π interaction between its gallic acid ester group and K28, while EGC damages the salt bridges through hydrophobic interactions with V39 and I41 as well as with I32, M35, and V40 located in the C-terminal hydrophobic core. This study demonstrates the pivotal role of the gallic acid ester group of EGCG in disrupting Aβ42 protofibril and provides atomic-level insights into the distinct mechanism by which EGCG and EGC disrupt Aβ protofibril, which could be useful for designing amyloid inhibitors.
中文翻译:

绿茶提取物EGCG和EGC在破坏Aβ42原纤维中显示出不同的机制。
淀粉样β(Aβ)纤维状聚集体是阿尔茨海默氏病(AD)的标志。分解预形成的原纤维或抑制Aβ聚集被认为是AD的治疗策略。越来越多的证据表明,绿茶提取物,表没食子儿茶素-3-没食子酸酯(EGCG,与EGC相比,含有额外的没食子酸酯基)和表没食子儿茶素(EGC),可以使Aβ原纤维解离并减弱Aβ毒性。但是,对潜在的分子机制了解甚少。在本文中,我们进行了微秒全原子分子动力学(MD)模拟,以研究EGCG / EGC对新的冷冻-EM解析的LS形Aβ42原纤维及其详细相互作用的影响。MD模拟表明EGCG和EGC均可破坏Aβ42原纤维和EGCG显示出比EGC更高的破坏能力。EGCG通过与残基H14 / Y10的π–π相互作用和与E11的氢键相互作用来破坏H6和E11之间的氢键,从而改变Aβ42原纤维的L形,而EGC通过插入疏水核中来重塑L形由A2,F4,L34和V36形成,并通过芳烃与H6 / Y10相互作用形成。EGCG破坏了K28侧链和A42 COO之间的盐桥–通过与A42的氢键相互作用以及其没食子酸酯基团和K28之间的阳离子-π相互作用,而EGC通过与V39和I41以及与C末端的I32,M35和V40的疏水相互作用破坏盐桥疏水核。这项研究证明了EGCG的没食子酸酯基团在破坏Aβ42的原纤维中的关键作用,并提供了原子级的见解,以了解EGCG和EGC破坏Aβ的原纤维,这对设计淀粉样蛋白抑制剂很有用。
更新日期:2020-05-22
中文翻译:

绿茶提取物EGCG和EGC在破坏Aβ42原纤维中显示出不同的机制。
淀粉样β(Aβ)纤维状聚集体是阿尔茨海默氏病(AD)的标志。分解预形成的原纤维或抑制Aβ聚集被认为是AD的治疗策略。越来越多的证据表明,绿茶提取物,表没食子儿茶素-3-没食子酸酯(EGCG,与EGC相比,含有额外的没食子酸酯基)和表没食子儿茶素(EGC),可以使Aβ原纤维解离并减弱Aβ毒性。但是,对潜在的分子机制了解甚少。在本文中,我们进行了微秒全原子分子动力学(MD)模拟,以研究EGCG / EGC对新的冷冻-EM解析的LS形Aβ42原纤维及其详细相互作用的影响。MD模拟表明EGCG和EGC均可破坏Aβ42原纤维和EGCG显示出比EGC更高的破坏能力。EGCG通过与残基H14 / Y10的π–π相互作用和与E11的氢键相互作用来破坏H6和E11之间的氢键,从而改变Aβ42原纤维的L形,而EGC通过插入疏水核中来重塑L形由A2,F4,L34和V36形成,并通过芳烃与H6 / Y10相互作用形成。EGCG破坏了K28侧链和A42 COO之间的盐桥–通过与A42的氢键相互作用以及其没食子酸酯基团和K28之间的阳离子-π相互作用,而EGC通过与V39和I41以及与C末端的I32,M35和V40的疏水相互作用破坏盐桥疏水核。这项研究证明了EGCG的没食子酸酯基团在破坏Aβ42的原纤维中的关键作用,并提供了原子级的见解,以了解EGCG和EGC破坏Aβ的原纤维,这对设计淀粉样蛋白抑制剂很有用。

































 京公网安备 11010802027423号
京公网安备 11010802027423号