当前位置:
X-MOL 学术
›
STEM CELLS
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Lineage-specific differentiation of osteogenic progenitors from pluripotent stem cells reveals the FGF1-RUNX2 association in neural crest-derived osteoprogenitors
STEM CELLS ( IF 4.0 ) Pub Date : 2020-06-09 , DOI: 10.1002/stem.3206 Fahad Kidwai 1 , Byron W H Mui 1 , Deepika Arora 1, 2 , Kulsum Iqbal 3 , Madison Hockaday 1 , Luis Fernandez de Castro Diaz 4 , Natasha Cherman 1 , Daniel Martin 5 , Vamsee D Myneni 6 , Moaz Ahmad 7 , Katarzyna Futrega 1 , Sania Ali 8 , Randall K Merling 1 , Dan S Kaufman 9 , Janice Lee 10 , Pamela G Robey 1
STEM CELLS ( IF 4.0 ) Pub Date : 2020-06-09 , DOI: 10.1002/stem.3206 Fahad Kidwai 1 , Byron W H Mui 1 , Deepika Arora 1, 2 , Kulsum Iqbal 3 , Madison Hockaday 1 , Luis Fernandez de Castro Diaz 4 , Natasha Cherman 1 , Daniel Martin 5 , Vamsee D Myneni 6 , Moaz Ahmad 7 , Katarzyna Futrega 1 , Sania Ali 8 , Randall K Merling 1 , Dan S Kaufman 9 , Janice Lee 10 , Pamela G Robey 1
Affiliation
|
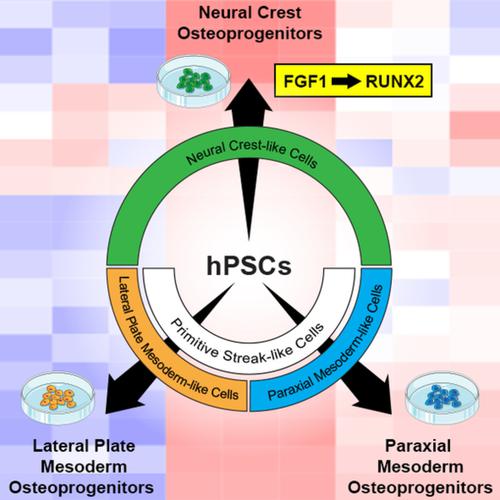
Human pluripotent stem cells (hPSCs) can provide a platform to model bone organogenesis and disease. To reflect the developmental process of the human skeleton, hPSC differentiation methods should include osteogenic progenitors (OPs) arising from three distinct embryonic lineages: the paraxial mesoderm, lateral plate mesoderm, and neural crest. Although OP differentiation protocols have been developed, the lineage from which they are derived, as well as characterization of their genetic and molecular differences, has not been well reported. Therefore, to generate lineage‐specific OPs from human embryonic stem cells and human induced pluripotent stem cells, we employed stepwise differentiation of paraxial mesoderm‐like cells, lateral plate mesoderm‐like cells, and neural crest‐like cells toward their respective OP subpopulation. Successful differentiation, confirmed through gene expression and in vivo assays, permitted the identification of transcriptomic signatures of all three cell populations. We also report, for the first time, high FGF1 levels in neural crest‐derived OPs—a notable finding given the critical role of fibroblast growth factors (FGFs) in osteogenesis and mineral homeostasis. Our results indicate that FGF1 influences RUNX2 levels, with concomitant changes in ERK1/2 signaling. Overall, our study further validates hPSCs' power to model bone development and disease and reveals new, potentially important pathways influencing these processes.
中文翻译:

来自多能干细胞的成骨祖细胞的谱系特异性分化揭示了神经嵴衍生的骨祖细胞中的 FGF1-RUNX2 关联
人类多能干细胞(hPSC)可以提供一个平台来模拟骨器官发生和疾病。为了反映人体骨骼的发育过程,hPSC 分化方法应包括来自三个不同胚胎谱系的成骨祖细胞 (OP):近轴中胚层、侧板中胚层和神经嵴。尽管OP分化方案已经开发出来,但它们的谱系以及它们的遗传和分子差异的特征尚未得到很好的报道。因此,为了从人胚胎干细胞和人诱导多能干细胞中产生谱系特异性OP,我们采用了近轴中胚层样细胞、侧板中胚层样细胞和神经嵴样细胞向各自OP亚群的逐步分化。通过基因表达和体内测定证实的成功分化,允许鉴定所有三个细胞群的转录组特征。我们还首次报告了神经嵴衍生的 OP 中 FGF1 水平较高——考虑到成纤维细胞生长因子 (FGF) 在成骨和矿物质稳态中的关键作用,这是一个值得注意的发现。我们的结果表明 FGF1 影响 RUNX2 水平,并伴随 ERK1/2 信号传导的变化。总体而言,我们的研究进一步验证了 hPSC 模拟骨骼发育和疾病的能力,并揭示了影响这些过程的新的、潜在的重要途径。
更新日期:2020-06-09
中文翻译:

来自多能干细胞的成骨祖细胞的谱系特异性分化揭示了神经嵴衍生的骨祖细胞中的 FGF1-RUNX2 关联
人类多能干细胞(hPSC)可以提供一个平台来模拟骨器官发生和疾病。为了反映人体骨骼的发育过程,hPSC 分化方法应包括来自三个不同胚胎谱系的成骨祖细胞 (OP):近轴中胚层、侧板中胚层和神经嵴。尽管OP分化方案已经开发出来,但它们的谱系以及它们的遗传和分子差异的特征尚未得到很好的报道。因此,为了从人胚胎干细胞和人诱导多能干细胞中产生谱系特异性OP,我们采用了近轴中胚层样细胞、侧板中胚层样细胞和神经嵴样细胞向各自OP亚群的逐步分化。通过基因表达和体内测定证实的成功分化,允许鉴定所有三个细胞群的转录组特征。我们还首次报告了神经嵴衍生的 OP 中 FGF1 水平较高——考虑到成纤维细胞生长因子 (FGF) 在成骨和矿物质稳态中的关键作用,这是一个值得注意的发现。我们的结果表明 FGF1 影响 RUNX2 水平,并伴随 ERK1/2 信号传导的变化。总体而言,我们的研究进一步验证了 hPSC 模拟骨骼发育和疾病的能力,并揭示了影响这些过程的新的、潜在的重要途径。































 京公网安备 11010802027423号
京公网安备 11010802027423号