Journal of Molecular Liquids ( IF 5.3 ) Pub Date : 2020-05-23 , DOI: 10.1016/j.molliq.2020.113411 Dawei Shang , Shaojuan Zeng , Xiangping Zhang , Xiaochun Zhang , Lu Bai , Haifeng Dong
|
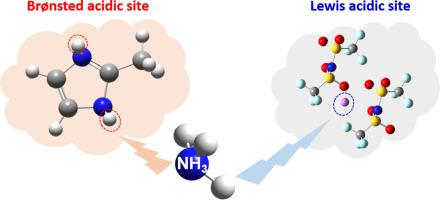
Ammonia (NH3) pollution poses a serious environmental threat. Ionic liquids (ILs) are regarded as potential solvents to treat NH3-containing gases because of their unique properties, including tunable structures and high affinity for NH3 molecules. In order to achieve an efficient and reversible absorption of NH3, a series of protic ionic liquids (PILs) with multiple active sites were designed by introducing acidic protons, as well as the Li+ ion into ILs, resulting in PILs with two acidic protons on cations ([2-Mim][NTf2] and [Im][NTf2]), as well as dual functionalised PILs with a Lewis acidic Li+-based anion and acidic protons on cations, namely, [2-Mim][Li(NTf2)2] and [Eim][Li(NTf2)2]. The NH3 absorption capacity of [Im][NTf2] was found to be 3.46 mol NH3/mol IL, which is the highest among the NH3 absorption capacities of all known nonmetallic ILs, and is ascribed to the hydrogen bonding interactions between the NH3 and PIL molecules. Furthermore, [2-Mim][Li(NTf2)2] exhibited the highest NH3 absorption capacity, with a value of 7.01 mol NH3/mol IL, which is about 30 times higher than that of conventional ILs. The superior NH3 absorption performance of the dual functionalised PILs is attributed to the combination of hydrogen bonding interactions between the protons and the NH3 molecules, and the formation of coordination complexes between the Li+-based anion and the NH3 molecules. Moreover, the ILs studied here exhibited excellent recyclability, indicating a great potential for their use in applications involving efficient and reversible NH3 absorption from NH3-containing industrial gases.
中文翻译:

具有质子和路易斯酸性位点的双重功能化离子液体对NH 3的高效可逆吸收
氨(NH 3)污染构成严重的环境威胁。离子液体(ILs)由于其独特的特性(包括可调结构和对NH 3分子的高亲和力)而被视为处理含NH 3气体的潜在溶剂。为了实现NH 3的有效和可逆吸收,通过将酸性质子以及Li +离子引入IL,设计了具有多个活性位的一系列质子离子液体(PIL),生成了带有两个酸性质子的PIL。阳离子([2-Mim] [NTf 2 ]和[Im] [NTf 2 ])以及具有路易斯酸性Li +的双官能化PIL的阳离子和酸性质子,即[2-Mim] [Li(NTf 2)2 ]和[Eim] [Li(NTf 2)2 ]。发现[Im] [NTf 2 ]的NH 3吸收容量为3.46 mol NH 3 / mol IL,在所有已知的非金属离子液体的NH 3吸收容量中是最高的,并且归因于它们之间的氢键相互作用NH 3和PIL分子。此外,[2-Mim] [Li(NTf 2)2 ]表现出最高的NH 3吸收容量,值为7.01 mol NH 3。/ mol IL,大约是传统IL的30倍。双官能化PIL的优异的NH 3吸收性能归因于质子与NH 3分子之间的氢键相互作用,以及基于Li +的阴离子与NH 3分子之间形成配位络合物。另外,在此研究的离子液体表现出优异的再利用性,这表明它们在涉及高效和可逆NH应用中使用的潜力很大3选自NH吸收3含工业气体。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号