Journal of Hazardous Materials ( IF 12.2 ) Pub Date : 2020-05-23 , DOI: 10.1016/j.jhazmat.2020.122955 Jun Li 1 , Yiming Lai 1 , Xianqing Zhu 1 , Qiang Liao 1 , Ao Xia 1 , Yun Huang 1 , Xun Zhu 1
|
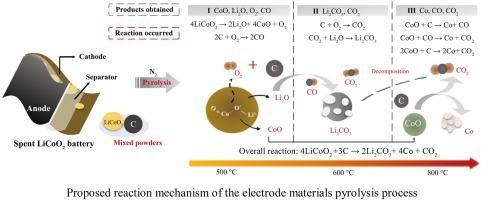
The spent lithium-ion batteries (LIBs) have potentially serious environmental hazards but contain various valuable metals. Pyrolysis has been preliminarily proven to be an efficient method to dispose spent LIBs and recycle valuable metals. However, the kinetics and reaction mechanism during this pyrolysis process still remain unclear. Therefore, in this study, the pyrolysis kinetics and reaction mechanism of a typical spent LIB (LiCoO2 battery) was investigated and revealed in depth. The results indicated that the reactions happened to the electrode materials (LiCoO2, C) were mainly in the range of 500−800 °C. Two iso-conversion methods (Kissinger–Akahira–Sunose model and Flynn–Wall–Ozawa model) could both well describe the pyrolysis process, and the corresponding activation energies obtained were 389.61 and 405.67 kJ/mol respectively. The physicochemical properties of the pyrolysis products were detailedly characterized to reveal the reaction mechanism. The pyrolysis reaction mechanism of the electrode materials was firstly proposed and divided into three stages: firstly, LiCoO2 was decomposed into CoO, O2 and Li2O; then Li2O reacted with CO2 to form Li2CO3; finally CoO was reduced and converted into Co. This study is expected to provide a comprehensive understanding of the pyrolysis kinetics and reaction mechanism during the spent LiCoO2 batteries recovery process.
中文翻译:

LiCoO2废电池回收过程中电极材料的热解动力学和反应机理。
废锂离子电池(LIB)具有潜在的严重环境危害,但包含各种贵重金属。初步证明热解是处理用过的LIB和回收贵金属的有效方法。然而,在该热解过程中的动力学和反应机理仍然不清楚。因此,在这项研究中,深入研究并揭示了典型的废LIB(LiCoO 2电池)的热解动力学和反应机理。结果表明,反应发生在电极材料(LiCoO 2,C)主要在500-800°C的范围内。两种同工转换方法(Kissinger–Akahira–Sunose模型和Flynn–Wall–Ozawa模型)都可以很好地描述热解过程,获得的相应活化能分别为389.61和405.67 kJ / mol。详细描述了热解产物的理化性质,以揭示反应机理。电极材料的热分解反应机理首先提出并分为三个阶段:首先,的LiCoO 2被分解成的CoO,O- 2和Li 2 O; 然后Li 2 O与CO 2反应形成Li 2 CO 3; 最终,CoO被还原并转化为Co。该研究有望提供对废LiCoO 2电池回收过程中热解动力学和反应机理的全面理解。































 京公网安备 11010802027423号
京公网安备 11010802027423号