Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2020-05-24 , DOI: 10.1016/j.bioorg.2020.103954 Lukuan Hou 1 , Zengzhi Liu 1 , Dongqi Yu 1 , Huayue Li 2 , Jianhua Ju 3 , Wenli Li 2
|
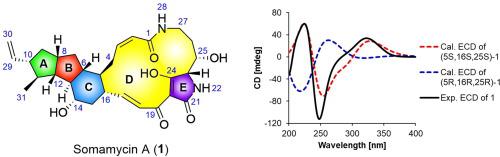
With a combined strategy of bioinformatics analysis, gene manipulation coupled with variation of growth conditions, the targeted activation of polycyclic tetramate macrolactams (PTMs) in the deepsea-derived Streptomyces somaliensis SCSIO ZH66 was conducted, which afforded a new (1) PTM, named somamycin A, along with three enol-type tetramic acid tautomers (2−4, somamycins B−D) of 10-epi-hydroxymaltophilin, 10-epi-maltophilin and 10-epi-HSAF, respectively. The structures of compounds 1−4 were elucidated by extensive spectroscopic analyses together with ECD calculations. Compound 1 exhibited notable growth inhibition against plant pathogenic fungi Fusarium oxysporum MHKW and Alternaria brassicae BCHB with the MIC values of 1.6 and 3.1 μg/mL, respectively, which were more potent than those of the positive control nystatin; and compounds 3 and 4 displayed moderate antifungal activities. Moreover, compounds 1−4 exhibited moderate cytotoxicity against the human cancer cell lines of HCT116 and K562.
中文翻译:

从深海衍生的索马里链霉菌SCSIO ZH66靶向分离新的多环四酸酯大内酰胺。
结合生物信息学分析,基因操作和生长条件变化的联合策略,在深海来源的索马里链霉菌SCSIO ZH66中进行了多环四酸酯大内酰胺(PTM)的靶向活化,从而提供了一种新的(1)PTM,名为索马霉素A,具有三个烯醇型特特拉姆酸互变异构体沿(2 - 4,somamycins B-d)10-外延-hydroxymaltophilin,10-外延-maltophilin和10-外延分别-HSAF。化合物的结构1 - 4被广泛光谱分析与ECD计算阐明在一起。化合物1表现出对植物显着的生长抑制致病真菌尖孢镰孢MHKW和孢甘蓝BCHB 1.6和3.1的MIC值μ克/毫升,分别均较阳性对照制霉菌素的更有效的; 化合物3和4表现出中等的抗真菌活性。此外,化合物1 - 4显示出适中的细胞毒性对HCT116和K562的人类癌细胞系。






























 京公网安备 11010802027423号
京公网安备 11010802027423号