当前位置:
X-MOL 学术
›
Cancer Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ferroptosis at the crossroads of infection, aging and cancer.
Cancer Science ( IF 4.5 ) Pub Date : 2020-05-21 , DOI: 10.1111/cas.14496 Shinya Toyokuni 1, 2, 3 , Izumi Yanatori 1 , Yingyi Kong 1 , Hao Zheng 1 , Yashiro Motooka 1 , Li Jiang 1
Cancer Science ( IF 4.5 ) Pub Date : 2020-05-21 , DOI: 10.1111/cas.14496 Shinya Toyokuni 1, 2, 3 , Izumi Yanatori 1 , Yingyi Kong 1 , Hao Zheng 1 , Yashiro Motooka 1 , Li Jiang 1
Affiliation
|
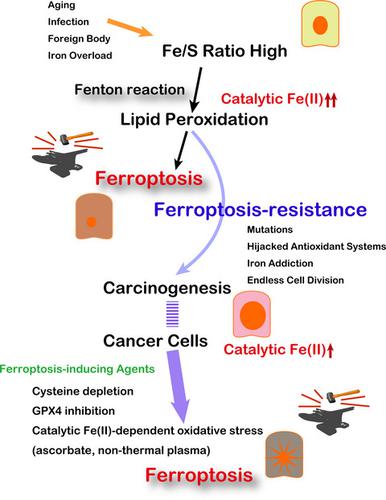
Despite significant developments and persistent efforts by scientists, cancer is one of the primary causes of human death worldwide. No form of life on Earth can survive without iron, although some species can live without oxygen. Iron presents a double‐edged sword. Excess iron is a risk for carcinogenesis, while its deficiency causes anemia, leading to oxygen shortage. Every cell is eventually destined to death, either through apoptosis or necrosis. Regulated necrosis is recognized in distinct forms. Ferroptosis is defined as catalytic Fe(II)‐dependent regulated necrosis accompanied by lipid peroxidation. The main observation was necrosis of fibrosarcoma cells through inhibition of cystine/glutamate antiporter with erastin, which reduced intracellular cysteine and, thus, glutathione levels. Our current understanding of ferroptosis is relative abundance of iron (catalytic Fe[II]) in comparison with sulfur (sulfhydryls). Thus, either excess iron or sulfur deficiency causes ferroptosis. Cell proliferation inevitably requires iron for DNA synthesis and energy production. Carcinogenesis is a process toward iron addiction with ferroptosis resistance. Conversely, ferroptosis is associated with aging and neurodegeneration. Ferroptosis of immune cells during infection is advantageous for infectious agents, whereas ferroptosis resistance incubates carcinogenic soil as excess iron. Cancer cells are rich in catalytic Fe(II). Directing established cancer cells to ferroptosis is a novel strategy for discovering cancer therapies. Appropriate iron regulation could be a tactic to reduce and delay carcinogenesis.
中文翻译:

处于感染,衰老和癌症十字路口的铁锈病。
尽管科学家取得了重大进展并做出了不懈的努力,但癌症仍是全世界人类死亡的主要原因之一。尽管有些物种没有氧气,但没有铁,地球上任何形式的生命都无法生存。铁呈现出一把双刃剑。铁过量会致癌,而铁缺乏会导致贫血,导致缺氧。每个细胞最终都会通过凋亡或坏死注定要死亡。调节坏死以不同形式被识别。Ferroptosis被定义为催化性Fe(II)依赖性坏死伴有脂质过氧化作用。主要观察结果是通过用Estin抑制胱氨酸/谷氨酸逆向转运蛋白,使纤维肉瘤细胞坏死,从而减少了细胞内半胱氨酸,从而降低了谷胱甘肽水平。我们目前对肥大病的理解是铁(催化性Fe [II])相对于硫(巯基)的相对丰度。因此,铁或硫的过量缺乏都会引起肥大症。细胞增殖不可避免地需要铁来合成DNA和产生能量。致癌作用是具有抗铁变性的铁成瘾过程。相反,肥大症与衰老和神经变性有关。感染期间免疫细胞的肥大化对于传染原是有利的,而肥大化的抗性则以过量的铁温育致癌土壤。癌细胞富含催化性Fe(II)。将已建立的癌细胞引导到肥大症是一种发现癌症疗法的新策略。适当的铁调节可能是减少和延迟致癌作用的策略。
更新日期:2020-05-21
中文翻译:

处于感染,衰老和癌症十字路口的铁锈病。
尽管科学家取得了重大进展并做出了不懈的努力,但癌症仍是全世界人类死亡的主要原因之一。尽管有些物种没有氧气,但没有铁,地球上任何形式的生命都无法生存。铁呈现出一把双刃剑。铁过量会致癌,而铁缺乏会导致贫血,导致缺氧。每个细胞最终都会通过凋亡或坏死注定要死亡。调节坏死以不同形式被识别。Ferroptosis被定义为催化性Fe(II)依赖性坏死伴有脂质过氧化作用。主要观察结果是通过用Estin抑制胱氨酸/谷氨酸逆向转运蛋白,使纤维肉瘤细胞坏死,从而减少了细胞内半胱氨酸,从而降低了谷胱甘肽水平。我们目前对肥大病的理解是铁(催化性Fe [II])相对于硫(巯基)的相对丰度。因此,铁或硫的过量缺乏都会引起肥大症。细胞增殖不可避免地需要铁来合成DNA和产生能量。致癌作用是具有抗铁变性的铁成瘾过程。相反,肥大症与衰老和神经变性有关。感染期间免疫细胞的肥大化对于传染原是有利的,而肥大化的抗性则以过量的铁温育致癌土壤。癌细胞富含催化性Fe(II)。将已建立的癌细胞引导到肥大症是一种发现癌症疗法的新策略。适当的铁调节可能是减少和延迟致癌作用的策略。































 京公网安备 11010802027423号
京公网安备 11010802027423号