Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy ( IF 4.3 ) Pub Date : 2020-05-21 , DOI: 10.1016/j.saa.2020.118516
Aya T Soudi 1 , Ola G Hussein 2 , Eman S Elzanfaly 1 , Hala E Zaazaa 1 , Mohamed Abdelkawy 2
|
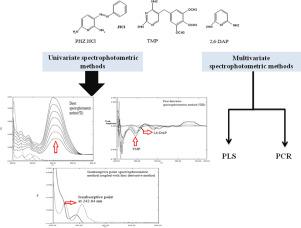
Three univariate and two multivariate spectrophotometric methods were developed and subsequently validated to determine phenazopyridine HCl (PHZ) and trimethoprim (TMP) in the presence of 2,6-Diaminopyridine (2,6-DAP). The first univariate method depends on direct determination of phenazopyridine by measuring its absorbance at 412 nm and performed in concentration range of 1.00–10.00 μg/mL. Then the contribution of phenazopyridine is removed by dividing the mixture spectrum with PHZ divisor (5 μg/mL) after that the constant is mathematically subtracted and finally the generated spectrum is multiplied with the PHZ divisor. These steps eliminate PHZ contribution and the recovered spectrum is that of TMP and 2,6-DAP only where different methods can be applied to determine TMP and 2,6-DAP through this binary mixture spectrum. The first method to determine both components depends on measuring both TMP and 2,6-DAP through their first derivative (1DD) spectra at 244.70 and 259.60 nm for TMP and 2,6-DAP, respectively with concentration ranges of 4.00–24.00 μg/mL TMP and 4.00–26.00 μg/mL 2,6-DAP. The second method depends on application of the isoabsorptive method which was used for TMP determination at its isoabsorptive point with 2,6-DAP at 242.64 nm with concentration range 1.00–20.00 μg/mL for TMP. The developed univariate methods were successfully applied to determine PHZ, TMP and PHZ impurity (2,6-DAP). Two multivariate methods were applied for determination of PHZ and TMP in presence of 2,6-DAP namely, Principle Component Regression (PCR) and Partial Least Squares (PLS). The results of the two models show that simultaneous determination of PHZ and TMP in presence of PHZ impurity can be performed in the concentration ranges of 6.00–14.00 μg/mL PHZ and 24.00–56.00 μg/mL TMP. All the proposed methods were successfully applied to analyze PHZ and TMP in pharmaceutical formulations without interference from the dosage form additives and the results were statistically compared with the reported method.,
中文翻译:

单变量和多变量分光光度法同时测定存在苯并吡啶盐酸盐杂质的同时测定盐酸苯并吡啶吡啶和甲氧苄啶-用单变量方法定量分析盐酸苯并吡啶吡啶杂质。
开发了三种单变量和两种多元分光光度法,随后在存在2,6-二氨基吡啶(2,6-DAP)的情况下验证了方法的有效性,可用于测定盐酸吩唑吡啶(PHZ)和甲氧苄啶(TMP)。第一个单变量方法取决于在1.00–10.00μg/ mL的浓度范围内通过测量其在412 nm处的吸光度直接测定苯并吡啶。然后,将混合物光谱除以PHZ除数(5μg/ mL),然后通过数学方式减去常数,最后将生成的光谱与PHZ除数相乘,从而除去苯并吡啶的作用。这些步骤消除了PHZ的影响,仅在可以应用不同方法通过该二元混合光谱测定TMP和2,6-DAP的情况下,回收的光谱才是TMP和2,6-DAP的光谱。1个DMP在244.70和259.60 nm处的TMP和2,6-DAP的浓度范围分别为4.00–24.00μg/ mL TMP和4.00–26.00μg/ mL的2,6-DAP。第二种方法取决于等吸收法的应用,该方法用于TMP测定的等吸收点,在262.64 nm处有2,6-DAP,TMP的浓度范围为1.00–20.00μg/ mL。所开发的单变量方法已成功应用于确定PHZ,TMP和PHZ杂质(2,6-DAP)。在2,6-DAP存在下,应用了两种多元方法测定PHZ和TMP,即主成分回归(PCR)和偏最小二乘(PLS)。两种模型的结果表明,可以在6.00-14.00μg/ mL PHZ和24.00-56.00μg/ mL TMP的浓度范围内同时测定存在PHZ杂质的PHZ和TMP。































 京公网安备 11010802027423号
京公网安备 11010802027423号