当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Aryl Nitrile Imines and Diazo Compounds. Formation of Indazole, Pyridine N-Imine, and 2-Pyridyldiazomethane from Tetrazoles.
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2020-05-20 , DOI: 10.1021/acs.joc.0c00773 Didier Bégué 1 , Alain Dargelos 1 , Curt Wentrup 2
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2020-05-20 , DOI: 10.1021/acs.joc.0c00773 Didier Bégué 1 , Alain Dargelos 1 , Curt Wentrup 2
Affiliation
|
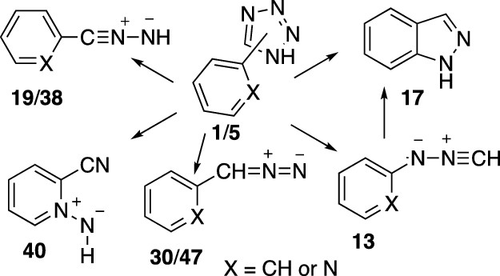
Both photolysis and flash vacuum pyrolysis (FVP) of tetrazoles (1/5) are known to generate nitrile imines (13, 19, and 38), which rearrange to 1H-diazirines, imidoylnitrenes, and carbodiimides. Moreover, FVP of 5-aryltetrazoles is a convenient source of aryldiazo compounds (30/47) and arylcarbenes, including pyridylcarbenes. The factors that determine which path is followed are poorly understood. Calculations at the density functional theory and CASPT2 levels now examine cyclization of N-phenylnitrile imine 13 to indazole 17. A corresponding cyclization of C-phenylnitrile imine 19 can also lead to indazole, but this reaction, which passes through a carbenic nitrile imine, requires a much higher activation energy and is therefore not competitive with the known rearrangements to phenyldiazirines, ring expansion to diazenylcycloheptatetraene, or a new, potential rearrangement to cyanoazepine. C-(2-Pyridyl)nitrile imine 38 is predicted to undergo a new rearrangement to cyanopyridine N-imide 40 with an activation energy of 43 kcal/mol. The experimental observation that 2-pyridyldiazomethane 47 is actually formed requires a reaction with an energy barrier below 43 kcal/mol. This is found in the H-transfer from the tetrazole ring in 5-(2-pyridyl)tetrazole to the pyridine ring with a subsequent formation of 1H-2-(diazomethylene)pyridine and elimination of N2 with a barrier of ca. 26 kcal/mol. This new, facile mechanism has not previously been considered.
中文翻译:

芳腈亚胺和重氮化合物。由四唑形成吲唑,吡啶N-亚胺和2-吡啶基重氮甲烷。
四唑(两个光解和闪热解真空(FVP)1 / 5)是已知的,以产生腈亚胺(13,19,和38),其重排成1个ħ -diazirines,imidoylnitrenes,和碳二亚胺。此外,5-aryltetrazoles FVP是aryldiazo化合物(的方便来源30 / 47)和arylcarbenes,包括pyridylcarbenes。决定遵循哪条路径的因素知之甚少。在密度泛函理论和CASPT2水平上的计算现在检查了N-苯基腈亚胺13向吲唑17的环化。C-苯基腈亚胺19的相应环化反应也可导致吲唑,但该反应通过羧腈亚胺,需要更高的活化能,因此与已知的重氮苯重氮竞争,环扩环为二氮烯基环庚二烯不竞争,或可能重新配置成氰基氮杂。C-(2-吡啶基)腈亚胺38预计将以43kcal / mol的活化能经历新的重排为氰基吡啶N-酰亚胺40。2-吡啶基重氮甲烷47的实验观察实际上形成的H 2 O需要具有低于43kcal / mol的能垒的反应。在5-(2-吡啶基)四唑中的四唑环到吡啶环的H-转移中,随后形成1 H -2-(重氮亚甲基)吡啶,并以约2的势垒消除N 2,发现了这一点。26 kcal / mol。以前从未考虑过这种新的简便机制。
更新日期:2020-06-19
中文翻译:

芳腈亚胺和重氮化合物。由四唑形成吲唑,吡啶N-亚胺和2-吡啶基重氮甲烷。
四唑(两个光解和闪热解真空(FVP)1 / 5)是已知的,以产生腈亚胺(13,19,和38),其重排成1个ħ -diazirines,imidoylnitrenes,和碳二亚胺。此外,5-aryltetrazoles FVP是aryldiazo化合物(的方便来源30 / 47)和arylcarbenes,包括pyridylcarbenes。决定遵循哪条路径的因素知之甚少。在密度泛函理论和CASPT2水平上的计算现在检查了N-苯基腈亚胺13向吲唑17的环化。C-苯基腈亚胺19的相应环化反应也可导致吲唑,但该反应通过羧腈亚胺,需要更高的活化能,因此与已知的重氮苯重氮竞争,环扩环为二氮烯基环庚二烯不竞争,或可能重新配置成氰基氮杂。C-(2-吡啶基)腈亚胺38预计将以43kcal / mol的活化能经历新的重排为氰基吡啶N-酰亚胺40。2-吡啶基重氮甲烷47的实验观察实际上形成的H 2 O需要具有低于43kcal / mol的能垒的反应。在5-(2-吡啶基)四唑中的四唑环到吡啶环的H-转移中,随后形成1 H -2-(重氮亚甲基)吡啶,并以约2的势垒消除N 2,发现了这一点。26 kcal / mol。以前从未考虑过这种新的简便机制。































 京公网安备 11010802027423号
京公网安备 11010802027423号