当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The Human Cytochrome c Domain-Swapped Dimer Facilitates Tight Regulation of Intrinsic Apoptosis.
Biochemistry ( IF 2.9 ) Pub Date : 2020-05-19 , DOI: 10.1021/acs.biochem.0c00326 Harmen B B Steele 1, 2 , Margaret M Elmer-Dixon 1, 2 , James T Rogan 1 , J B Alexander Ross 1, 2 , Bruce E Bowler 1, 2
Biochemistry ( IF 2.9 ) Pub Date : 2020-05-19 , DOI: 10.1021/acs.biochem.0c00326 Harmen B B Steele 1, 2 , Margaret M Elmer-Dixon 1, 2 , James T Rogan 1 , J B Alexander Ross 1, 2 , Bruce E Bowler 1, 2
Affiliation
|
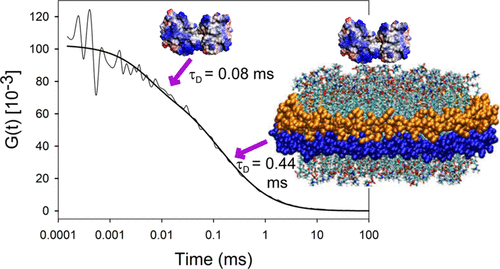
Oxidation of cardiolipin (CL) by cytochrome c (cytc) has been proposed to initiate the intrinsic pathway of apoptosis. Domain-swapped dimer (DSD) conformations of cytc have been reported both by our laboratory and by others. The DSD is an alternate conformer of cytc that could oxygenate CL early in apoptosis. We demonstrate here that the cytc DSD has a set of properties that would provide tighter regulation of the intrinsic pathway. We show that the human DSD is kinetically more stable than horse and yeast DSDs. Circular dichroism data indicate that the DSD has a less asymmetric heme environment, similar to that seen when the monomeric protein binds to CL vesicles at high lipid:protein ratios. The dimer undergoes the alkaline conformational transition near pH 7.0, 2.5 pH units lower than that of the monomer. Data from fluorescence correlation spectroscopy and fluorescence anisotropy suggest that the alkaline transition of the DSD may act as a switch from a high affinity for CL nanodiscs at pH 7.4 to a much lower affinity at pH 8.0. Additionally, the peroxidase activity of the human DSD increases 7-fold compared to that of the monomer at pH 7 and 8, but by 14-fold at pH 6 when mixed Met80/H2O ligation replaces the lysine ligation of the alkaline state. We also present data that indicate that cytc binding shows a cooperative effect as the concentration of cytc is increased. The DSD appears to have evolved into a pH-inducible switch that provides a means to control activation of apoptosis near pH 7.0.
中文翻译:

人细胞色素 c 结构域交换二聚体有助于严格调节内在细胞凋亡。
细胞色素c (cyt c ) 对心磷脂 (CL) 的氧化被认为可以启动细胞凋亡的内在途径。我们的实验室和其他实验室都报道了 cyt c的结构域交换二聚体 (DSD) 构象。 DSD 是细胞色素c的替代构象异构体,可以在细胞凋亡早期氧化 CL。我们在此证明 cyt c DSD 具有一组特性,可以对内在途径提供更严格的调节。我们证明人类 DSD 在动力学上比马和酵母 DSD 更稳定。圆二色性数据表明,DSD 具有较少不对称的血红素环境,类似于单体蛋白以高脂质:蛋白质比率与 CL 囊泡结合时所看到的情况。二聚体在pH 7.0附近发生碱性构象转变,比单体低2.5个pH单位。荧光相关光谱和荧光各向异性的数据表明,DSD 的碱性转变可能起到从 pH 7.4 时对 CL 纳米圆盘的高亲和力到 pH 8.0 时低得多的亲和力的转换作用。此外,与单体相比,在pH 7和8下,人DSD的过氧化物酶活性增加了7倍,但当混合Met80/H 2 O连接取代碱性状态的赖氨酸连接时,在pH 6下增加了14倍。我们还提供的数据表明,随着细胞色素c浓度的增加,细胞色素c结合显示出协同效应。 DSD 似乎已经演变成一种 pH 诱导开关,提供了一种在 pH 7.0 附近控制细胞凋亡激活的方法。
更新日期:2020-05-19
中文翻译:

人细胞色素 c 结构域交换二聚体有助于严格调节内在细胞凋亡。
细胞色素c (cyt c ) 对心磷脂 (CL) 的氧化被认为可以启动细胞凋亡的内在途径。我们的实验室和其他实验室都报道了 cyt c的结构域交换二聚体 (DSD) 构象。 DSD 是细胞色素c的替代构象异构体,可以在细胞凋亡早期氧化 CL。我们在此证明 cyt c DSD 具有一组特性,可以对内在途径提供更严格的调节。我们证明人类 DSD 在动力学上比马和酵母 DSD 更稳定。圆二色性数据表明,DSD 具有较少不对称的血红素环境,类似于单体蛋白以高脂质:蛋白质比率与 CL 囊泡结合时所看到的情况。二聚体在pH 7.0附近发生碱性构象转变,比单体低2.5个pH单位。荧光相关光谱和荧光各向异性的数据表明,DSD 的碱性转变可能起到从 pH 7.4 时对 CL 纳米圆盘的高亲和力到 pH 8.0 时低得多的亲和力的转换作用。此外,与单体相比,在pH 7和8下,人DSD的过氧化物酶活性增加了7倍,但当混合Met80/H 2 O连接取代碱性状态的赖氨酸连接时,在pH 6下增加了14倍。我们还提供的数据表明,随着细胞色素c浓度的增加,细胞色素c结合显示出协同效应。 DSD 似乎已经演变成一种 pH 诱导开关,提供了一种在 pH 7.0 附近控制细胞凋亡激活的方法。















































 京公网安备 11010802027423号
京公网安备 11010802027423号