当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Experimental Measurement of CO2 Solubility in Aqueous Na2SO4 Solution at Temperatures between 303.15 and 423.15 K and Pressures up to 20 MPa
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2020-05-18 , DOI: 10.1021/acs.jced.0c00230 Pedro F. dos Santos 1 , Laurent André 2, 3 , Marion Ducousso 1 , François Contamine 1 , Pierre Cézac 1
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2020-05-18 , DOI: 10.1021/acs.jced.0c00230 Pedro F. dos Santos 1 , Laurent André 2, 3 , Marion Ducousso 1 , François Contamine 1 , Pierre Cézac 1
Affiliation
|
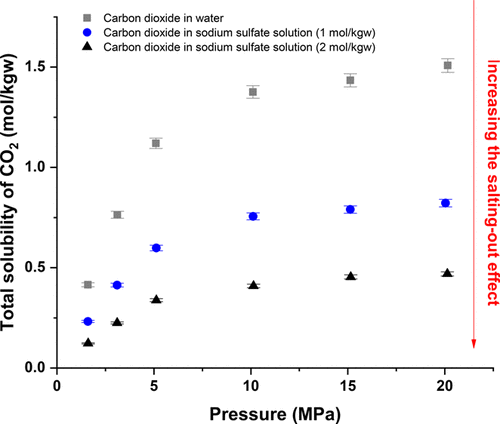
The geological storage of carbon dioxide CO2 in deep saline aquifers has been gaining interest to reduce greenhouse gas emissions. Thus, the number of studies focusing on the solubility of CO2 in brines at high temperature and high pressure has been increasing in the last decades, but information remains scarce particularly at high salt molality. In this work, an analytical method was developed for experimental measurement of CO2 solubility in sodium sulfate (Na2SO4) solutions (1 and 2 mol/kg) at high pressures (1.5–20 MPa) and temperatures (303.15, 323.15, 373.15, and 423.15 K). Forty-eight experimental points of CO2 solubility are reported here. Solubility has been shown to increase with pressure evolution and decrease with temperature and Na2SO4 concentrations evolution. A discussion is proposed about the salting-out effect in Na2SO4 solutions, and a comparison is made with the same effect in aqueous systems containing sodium chloride (NaCl) and calcium chloride (CaCl2).
中文翻译:

在303.15至423.15 K和最高20 MPa的压力下,Na 2 SO 4水溶液中CO 2溶解度的实验测量
二氧化碳CO的地质储存2在深盐水层已获得利益,以减少温室气体的排放。因此,在过去的几十年中,致力于高温和高压下的CO 2在盐水中的溶解度的研究数量一直在增加,但是信息仍然很少,特别是在高盐摩尔浓度下。在这项工作中,开发了一种分析方法,用于在高压(1.5–20 MPa)和高温(303.15,323.15,1)的条件下测量硫酸钠(Na 2 SO 4)溶液(1和2 mol / kg)中CO 2的溶解度。373.15和423.15 K)。CO 2的48个实验点溶解度在这里报道。已显示溶解度随压力的变化而增加,而随温度和Na 2 SO 4浓度的变化而降低。提出了关于在Na 2 SO 4溶液中的盐析作用的讨论,并且在包含氯化钠(NaCl)和氯化钙(CaCl 2)的水性体系中进行了比较,发现具有相同的作用。
更新日期:2020-05-18
中文翻译:

在303.15至423.15 K和最高20 MPa的压力下,Na 2 SO 4水溶液中CO 2溶解度的实验测量
二氧化碳CO的地质储存2在深盐水层已获得利益,以减少温室气体的排放。因此,在过去的几十年中,致力于高温和高压下的CO 2在盐水中的溶解度的研究数量一直在增加,但是信息仍然很少,特别是在高盐摩尔浓度下。在这项工作中,开发了一种分析方法,用于在高压(1.5–20 MPa)和高温(303.15,323.15,1)的条件下测量硫酸钠(Na 2 SO 4)溶液(1和2 mol / kg)中CO 2的溶解度。373.15和423.15 K)。CO 2的48个实验点溶解度在这里报道。已显示溶解度随压力的变化而增加,而随温度和Na 2 SO 4浓度的变化而降低。提出了关于在Na 2 SO 4溶液中的盐析作用的讨论,并且在包含氯化钠(NaCl)和氯化钙(CaCl 2)的水性体系中进行了比较,发现具有相同的作用。

































 京公网安备 11010802027423号
京公网安备 11010802027423号