当前位置:
X-MOL 学术
›
Chem. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Understanding the Origin of Enhanced Li-Ion Transport in Nanocrystalline Argyrodite-Type Li6PS5I.
Chemistry of Materials ( IF 7.2 ) Pub Date : 2020-05-19 , DOI: 10.1021/acs.chemmater.0c01367 Marina Brinek 1 , Caroline Hiebl 1 , H Martin R Wilkening 1
Chemistry of Materials ( IF 7.2 ) Pub Date : 2020-05-19 , DOI: 10.1021/acs.chemmater.0c01367 Marina Brinek 1 , Caroline Hiebl 1 , H Martin R Wilkening 1
Affiliation
|
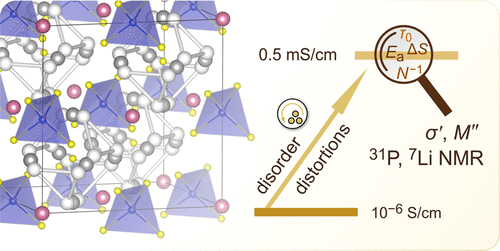
Argyrodite-type Li6PS5X (X = Cl, Br) compounds are considered to act as powerful ionic conductors in next-generation all-solid-state lithium batteries. In contrast to Li6PS5Br and Li6PS5Cl compounds showing ionic conductivities on the order of several mS cm–1, the iodine compound Li6PS5I turned out to be a poor ionic conductor. This difference has been explained by anion site disorder in Li6PS5Br and Li6PS5Cl leading to facile through-going, that is, long-range ion transport. In the structurally ordered compound, Li6PS5I, long-range ion transport is, however, interrupted because the important intercage Li jump-diffusion pathway, enabling the ions to diffuse over long distances, is characterized by higher activation energy than that in the sibling compounds. Here, we introduced structural disorder in the iodide by soft mechanical treatment and took advantage of a high-energy planetary mill to prepare nanocrystalline Li6PS5I. A milling time of only 120 min turned out to be sufficient to boost ionic conductivity by 2 orders of magnitude, reaching σtotal = 0.5 × 10–3 S cm–1. We followed this noticeable increase in ionic conductivity by broad-band conductivity spectroscopy and 7Li nuclear magnetic relaxation. X-ray powder diffraction and high-resolution 6Li, 31P MAS NMR helped characterize structural changes and the extent of disorder introduced. Changes in attempt frequency, activation entropy, and charge carrier concentration seem to be responsible for this increase.
中文翻译:

了解纳米晶Argyrodite型Li6PS5I中增强的锂离子运输的起源。
Argyrodite型Li 6 PS 5 X(X = Cl,Br)化合物被认为是下一代全固态锂电池中强大的离子导体。与Li 6 PS 5 Br和Li 6 PS 5 Cl化合物显示出数mS cm –1的离子电导率相反,碘化合物Li 6 PS 5 I证明是不良的离子导体。Li 6 PS 5 Br和Li 6 PS 5中的阴离子位点紊乱可以解释这种差异。Cl导致容易通过,即远程离子传输。然而,在结构有序的化合物Li 6 PS 5 I中,长距离离子传输被中断了,因为重要的键间Li跃迁扩散途径使离子能够长距离扩散,其特征在于活化能比Li 2 PS高。兄弟姐妹的化合物。在这里,我们引入了结构紊乱的碘化物通过软机械处理,并采取了高能行星式磨机的优点,制备纳米晶体栗6 PS 5的仅120分钟I.甲研磨时间原来是足以通过2以提高离子导电性数量级,达到σ总= 0.5×10 –3 S cm –1。我们通过宽带电导率光谱法和7 Li核磁弛豫跟踪了离子电导率的这种显着增加。X射线粉末衍射和高分辨率的6 Li,31 P MAS NMR有助于表征结构变化和引入的无序程度。尝试频率,激活熵和载流子浓度的变化似乎是造成这种增加的原因。
更新日期:2020-05-19
中文翻译:

了解纳米晶Argyrodite型Li6PS5I中增强的锂离子运输的起源。
Argyrodite型Li 6 PS 5 X(X = Cl,Br)化合物被认为是下一代全固态锂电池中强大的离子导体。与Li 6 PS 5 Br和Li 6 PS 5 Cl化合物显示出数mS cm –1的离子电导率相反,碘化合物Li 6 PS 5 I证明是不良的离子导体。Li 6 PS 5 Br和Li 6 PS 5中的阴离子位点紊乱可以解释这种差异。Cl导致容易通过,即远程离子传输。然而,在结构有序的化合物Li 6 PS 5 I中,长距离离子传输被中断了,因为重要的键间Li跃迁扩散途径使离子能够长距离扩散,其特征在于活化能比Li 2 PS高。兄弟姐妹的化合物。在这里,我们引入了结构紊乱的碘化物通过软机械处理,并采取了高能行星式磨机的优点,制备纳米晶体栗6 PS 5的仅120分钟I.甲研磨时间原来是足以通过2以提高离子导电性数量级,达到σ总= 0.5×10 –3 S cm –1。我们通过宽带电导率光谱法和7 Li核磁弛豫跟踪了离子电导率的这种显着增加。X射线粉末衍射和高分辨率的6 Li,31 P MAS NMR有助于表征结构变化和引入的无序程度。尝试频率,激活熵和载流子浓度的变化似乎是造成这种增加的原因。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号