当前位置:
X-MOL 学术
›
ACS Appl. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Revealing In Situ Li Metal Anode Surface Evolution upon Exposure to CO2 Using Ambient Pressure X-Ray Photoelectron Spectroscopy.
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2020-05-19 , DOI: 10.1021/acsami.0c04282 Ane Etxebarria 1, 2 , Dong-Jin Yun 3, 4 , Monika Blum 4, 5 , Yifan Ye 4, 5, 6 , Meiling Sun 4 , Kyung-Jae Lee 4, 7 , Hongyang Su 4, 8 , Miguel Ángel Muñoz-Márquez 1 , Philip N Ross 9 , Ethan J Crumlin 4, 5
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2020-05-19 , DOI: 10.1021/acsami.0c04282 Ane Etxebarria 1, 2 , Dong-Jin Yun 3, 4 , Monika Blum 4, 5 , Yifan Ye 4, 5, 6 , Meiling Sun 4 , Kyung-Jae Lee 4, 7 , Hongyang Su 4, 8 , Miguel Ángel Muñoz-Márquez 1 , Philip N Ross 9 , Ethan J Crumlin 4, 5
Affiliation
|
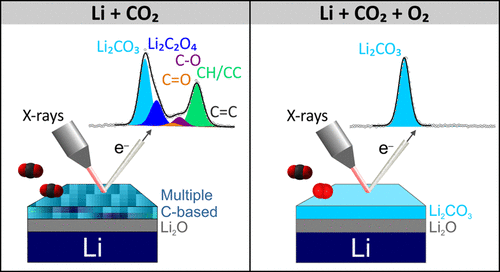
Because they deliver outstanding energy density, next-generation lithium metal batteries (LMBs) are essential to the advancement of both electric mobility and portable electronic devices. However, the high reactivity of metallic lithium surfaces leads to the low electrochemical performance of many secondary batteries. Besides, Li deposition is not uniform, which has been attributed to the low ionic conductivity of the anode surface. In particular, lithium exposure to CO2 gas is considered detrimental due to the formation of carbonate on the solid electrolyte interphase (SEI). In this work, we explored the interaction of Li metal with CO2 gas as a function of time using ambient pressure X-ray photoelectron spectroscopy to clarify the reaction pathway and main intermediates involved in the process during which oxalate formation has been detected. Furthermore, when O2 gas is part of the surrounding environment with CO2 gas, the reaction pathway is bypassed to directly promote carbonate as a single product.
中文翻译:

使用环境压力X射线光电子能谱揭示暴露于CO2后原位锂金属阳极表面的演变。
由于它们具有出色的能量密度,因此下一代锂金属电池(LMB)对于电动汽车和便携式电子设备的发展至关重要。然而,金属锂表面的高反应性导致许多二次电池的低电化学性能。此外,Li沉积不均匀,这归因于阳极表面的低离子电导率。特别地,由于在固体电解质界面(SEI)上形成碳酸盐,锂暴露于CO 2气体被认为是有害的。在这项工作中,我们探索了锂金属与CO 2的相互作用使用环境压力X射线光电子能谱法分析气体作为时间的函数,以澄清检测到草酸盐形成过程中的反应途径和主要中间体。另外,当O 2气体与CO 2气体一起成为周围环境的一部分时,反应路径被旁路以直接促进碳酸盐作为单一产物。
更新日期:2020-05-19
中文翻译:

使用环境压力X射线光电子能谱揭示暴露于CO2后原位锂金属阳极表面的演变。
由于它们具有出色的能量密度,因此下一代锂金属电池(LMB)对于电动汽车和便携式电子设备的发展至关重要。然而,金属锂表面的高反应性导致许多二次电池的低电化学性能。此外,Li沉积不均匀,这归因于阳极表面的低离子电导率。特别地,由于在固体电解质界面(SEI)上形成碳酸盐,锂暴露于CO 2气体被认为是有害的。在这项工作中,我们探索了锂金属与CO 2的相互作用使用环境压力X射线光电子能谱法分析气体作为时间的函数,以澄清检测到草酸盐形成过程中的反应途径和主要中间体。另外,当O 2气体与CO 2气体一起成为周围环境的一部分时,反应路径被旁路以直接促进碳酸盐作为单一产物。































 京公网安备 11010802027423号
京公网安备 11010802027423号