Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Protein Fibrils Formed by Rationally Designed α-Helical Peptides.
Langmuir ( IF 3.7 ) Pub Date : 2020-05-17 , DOI: 10.1021/acs.langmuir.0c00370 Xiangyu Sun 1 , Luhua Lai 1, 2
Langmuir ( IF 3.7 ) Pub Date : 2020-05-17 , DOI: 10.1021/acs.langmuir.0c00370 Xiangyu Sun 1 , Luhua Lai 1, 2
Affiliation
|
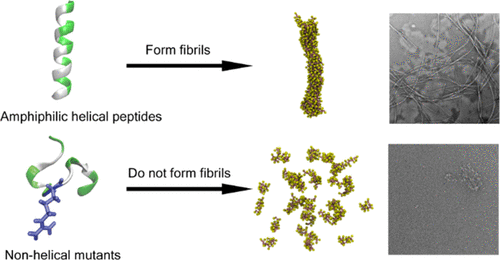
Fibrillar structures of proteins play essential roles in normal life events as well as diseases. It is of great importance to understand the principles by which proteins organize into fibrils. Here, we report a rationally designed α-helical peptide that can aggregate into fibrils. Mutation studies indicate that the helicity of the peptide is crucial for fibril formation. Multiscale molecular dynamics simulations demonstrated that the peptide may assemble in a quasiregular pattern, which is different from both the coiled coil and cross-α structures reported before. Our study provides a new helical peptide design that produces a fibrillar structure and contributes to the understanding of fibrillar structures formed by α-helices.
中文翻译:

由合理设计的α-螺旋肽形成的蛋白原纤维。
蛋白质的纤维状结构在正常的生活事件和疾病中起着至关重要的作用。了解蛋白质组织成原纤维的原理非常重要。在这里,我们报告了一个合理设计的α-螺旋肽,它可以聚集成原纤维。突变研究表明,该肽的螺旋结构对于原纤维形成至关重要。多尺度分子动力学模拟表明,该肽可能以准规则的模式组装,这与之前报道的卷曲螺旋和交叉α结构不同。我们的研究提供了一种新的螺旋肽设计,该设计可产生原纤维结构,并有助于理解由α-螺旋形成的原纤维结构。
更新日期:2020-05-17
中文翻译:

由合理设计的α-螺旋肽形成的蛋白原纤维。
蛋白质的纤维状结构在正常的生活事件和疾病中起着至关重要的作用。了解蛋白质组织成原纤维的原理非常重要。在这里,我们报告了一个合理设计的α-螺旋肽,它可以聚集成原纤维。突变研究表明,该肽的螺旋结构对于原纤维形成至关重要。多尺度分子动力学模拟表明,该肽可能以准规则的模式组装,这与之前报道的卷曲螺旋和交叉α结构不同。我们的研究提供了一种新的螺旋肽设计,该设计可产生原纤维结构,并有助于理解由α-螺旋形成的原纤维结构。






























 京公网安备 11010802027423号
京公网安备 11010802027423号