当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Plasma-Catalytic Ammonia Synthesis beyond the Equilibrium Limit
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-05-18 , DOI: 10.1021/acscatal.0c00684 Prateek Mehta 1 , Patrick M. Barboun 1 , Yannick Engelmann 2 , David B. Go 1, 3 , Annemie Bogaerts 2 , William F. Schneider 1 , Jason C. Hicks 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-05-18 , DOI: 10.1021/acscatal.0c00684 Prateek Mehta 1 , Patrick M. Barboun 1 , Yannick Engelmann 2 , David B. Go 1, 3 , Annemie Bogaerts 2 , William F. Schneider 1 , Jason C. Hicks 1
Affiliation
|
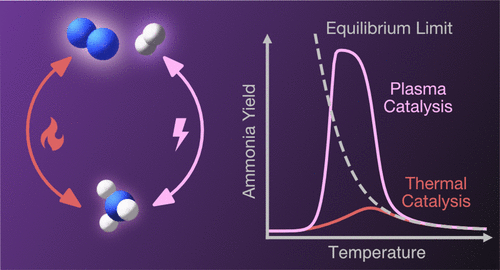
We explore the consequences of nonthermal plasma-activation on product yields in catalytic ammonia synthesis, a reaction that is equilibrium-limited at elevated temperatures. We employ a minimal microkinetic model that incorporates the influence of plasma-activation on N2 dissociation rates to predict NH3 yields into and across the equilibrium-limited regime. NH3 yields are predicted to exceed bulk thermodynamic equilibrium limits on materials that are thermal-rate-limited by N2 dissociation. In all cases, yields revert to bulk equilibrium at temperatures at which thermal reaction rates exceed plasma-activated ones. Beyond-equilibrium NH3 yields are observed in a packed bed dielectric barrier discharge reactor and exhibit sensitivity to catalytic material choice in a way consistent with model predictions. The approach and results highlight the opportunity to exploit synergies between nonthermal plasmas and catalysts to affect transformations at conditions inaccessible through thermal routes.
中文翻译:

超出平衡极限的等离子体催化氨合成
我们探索催化氨合成中非热等离子体活化对产物收率的影响,该反应在升高的温度下受到平衡限制。我们采用最小的微动力学模型,该模型结合了等离子体活化对N 2解离速率的影响,以预测进入和穿过平衡受限机制的NH 3收率。NH 3的产率预计会超过受N 2分解热速率限制的材料的整体热力学平衡极限。在所有情况下,在热反应速率超过等离子体活化速率的温度下,产率都恢复到整体平衡。超越平衡NH 3在填充床介质阻挡放电反应器中观察到产率,并且以与模型预测一致的方式表现出对催化材料选择的敏感性。该方法和结果突显了利用非热等离子体与催化剂之间的协同作用来影响通过热途径无法达到的条件下转化的机会。
更新日期:2020-06-19
中文翻译:

超出平衡极限的等离子体催化氨合成
我们探索催化氨合成中非热等离子体活化对产物收率的影响,该反应在升高的温度下受到平衡限制。我们采用最小的微动力学模型,该模型结合了等离子体活化对N 2解离速率的影响,以预测进入和穿过平衡受限机制的NH 3收率。NH 3的产率预计会超过受N 2分解热速率限制的材料的整体热力学平衡极限。在所有情况下,在热反应速率超过等离子体活化速率的温度下,产率都恢复到整体平衡。超越平衡NH 3在填充床介质阻挡放电反应器中观察到产率,并且以与模型预测一致的方式表现出对催化材料选择的敏感性。该方法和结果突显了利用非热等离子体与催化剂之间的协同作用来影响通过热途径无法达到的条件下转化的机会。






























 京公网安备 11010802027423号
京公网安备 11010802027423号