当前位置:
X-MOL 学术
›
Adv. Funct. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Building Artificial Solid‐Electrolyte Interphase with Uniform Intermolecular Ionic Bonds toward Dendrite‐Free Lithium Metal Anodes
Advanced Functional Materials ( IF 18.5 ) Pub Date : 2020-05-14 , DOI: 10.1002/adfm.202002414 Zhijie Wang 1, 2 , Yanyan Wang 1, 2 , Zihe Zhang 3 , Xiaowei Chen 4 , Wilford Lie 5 , Yan‐Bing He 6 , Zhen Zhou 3 , Guanglin Xia 1, 7 , Zaiping Guo 1, 2
Advanced Functional Materials ( IF 18.5 ) Pub Date : 2020-05-14 , DOI: 10.1002/adfm.202002414 Zhijie Wang 1, 2 , Yanyan Wang 1, 2 , Zihe Zhang 3 , Xiaowei Chen 4 , Wilford Lie 5 , Yan‐Bing He 6 , Zhen Zhou 3 , Guanglin Xia 1, 7 , Zaiping Guo 1, 2
Affiliation
|
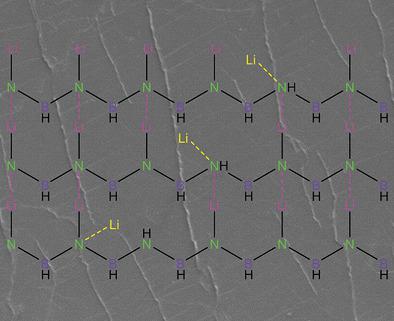
Li metal has been widely regarded as a promising anode for next‐generation batteries due to its high theoretical capacity and low electrochemical potential. The unstable solid‐electrolyte interphase (SEI) and uncontrollable dendrite growth, however, incur severe safety hazards and hamper the practical application of Li metal anodes. Herein, an advanced artificial SEI layer constructed by [LiNBH]n chains, which are crosslinked and self‐reinforced by their intermolecular LiN ionic bonds, is designed to comprehensively stabilize Li metal anodes on a molecular level. Benefiting from its polymer‐like structure, the [LiNBH]n layer is flexible and effectively tolerates the volume change of Li metal anodes. In addition, this layer with high polarity in its structure, helps to regulate the homogeneous distribution of the Li+ flux on Li electrodes via the further formation of LiN bonds. The designed [LiNBH]n layer is electrically nonconductive but highly ionically conductive, thus facilitating Li+ diffusion and confining Li deposition beneath the layer. Therefore, under the protection of the [LiNBH]n layer, the Li metal anodes exhibit stable cycling at a 3 mA cm−2 for more than 700 h, and the full cells with high lithium iron phosphate and sulfur cathodes mass loading also present excellent cycling stability.
中文翻译:

建立具有均匀分子间离子键的无树枝状锂金属阳极的人工固体电解质中间相
锂金属由于其高理论容量和低电化学势而被广泛认为是下一代电池的有希望的负极。然而,不稳定的固体电解质中间相(SEI)和无法控制的枝晶生长会带来严重的安全隐患,并阻碍锂金属阳极的实际应用。本文中,由[LiNBH] n 链构建的先进人工SEI层旨在通过分子间的LiN离子键交联并自我增强,旨在在分子水平上全面稳定Li金属阳极。得益于其类似聚合物的结构,[LiNBH] n 该层是柔性的并且有效地耐受锂金属阳极的体积变化。此外,这种结构中具有高极性的层通过进一步形成LiN键有助于调节Li +助焊剂在Li电极上的均匀分布。设计的[LiNBH] n层不导电,但离子导电性很高,因此有利于Li +扩散,并限制了Li在该层下的沉积。因此,在[LiNBH] n层的保护下,Li金属阳极在3 mA cm -2下表现出稳定的循环 在超过700小时的时间内,具有高磷酸铁锂和硫阴极质量负载的完整电池还具有出色的循环稳定性。
更新日期:2020-07-23
中文翻译:

建立具有均匀分子间离子键的无树枝状锂金属阳极的人工固体电解质中间相
锂金属由于其高理论容量和低电化学势而被广泛认为是下一代电池的有希望的负极。然而,不稳定的固体电解质中间相(SEI)和无法控制的枝晶生长会带来严重的安全隐患,并阻碍锂金属阳极的实际应用。本文中,由[LiNBH] n 链构建的先进人工SEI层旨在通过分子间的LiN离子键交联并自我增强,旨在在分子水平上全面稳定Li金属阳极。得益于其类似聚合物的结构,[LiNBH] n 该层是柔性的并且有效地耐受锂金属阳极的体积变化。此外,这种结构中具有高极性的层通过进一步形成LiN键有助于调节Li +助焊剂在Li电极上的均匀分布。设计的[LiNBH] n层不导电,但离子导电性很高,因此有利于Li +扩散,并限制了Li在该层下的沉积。因此,在[LiNBH] n层的保护下,Li金属阳极在3 mA cm -2下表现出稳定的循环 在超过700小时的时间内,具有高磷酸铁锂和硫阴极质量负载的完整电池还具有出色的循环稳定性。































 京公网安备 11010802027423号
京公网安备 11010802027423号