当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
One-Pot, Multicomponent, Diastereoselective, Green Synthesis of 3,4-Dihydro-2H-benzo[b][1,4]oxazine Analogues.
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2020-05-14 , DOI: 10.1021/acs.joc.0c00463 Narva Deshwar Kushwaha 1 , Babita Kushwaha 1 , Rajshekhar Karpoormath 1 , Mavela Cleopus Mahlalela 1 , Suraj Raosaheb Shinde 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2020-05-14 , DOI: 10.1021/acs.joc.0c00463 Narva Deshwar Kushwaha 1 , Babita Kushwaha 1 , Rajshekhar Karpoormath 1 , Mavela Cleopus Mahlalela 1 , Suraj Raosaheb Shinde 1
Affiliation
|
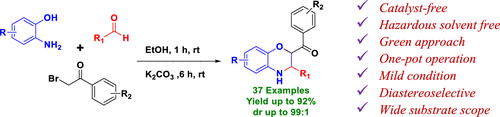
A novel green and efficient catalyst-free, mild one-pot, multicomponent synthetic strategy has been developed to construct substituted 3,4-dihydro-2H-benzo[b][1,4]oxazine. This reaction proceeds via in situ formation of Schiff-base followed by base mediated alkylation with phenacyl bromide/substituted phenacyl bromide, finally leading to intramolecular cyclization to give a mixture of diastereomers with excellent diastereoselectivity (up to dr = 99:1), which were isolated as a single diastereomer in moderate to excellent yields (41–92%). Besides, this new versatile methodology provides a wide scope for the synthesis of different functionally substituted benzoxazine scaffolds and can be further exploited as building blocks for the synthesis of multifaceted molecular structures, especially for pharmaceutical applications.
中文翻译:

一锅多组分非对映选择性绿色合成3,4-二氢-2H-苯并[b] [1,4]恶嗪类似物。
已开发出一种新颖的绿色高效无催化剂,温和的单锅多组分合成策略,以构建取代的3,4-二氢-2 H-苯并[ b]] [1,4]恶嗪。该反应通过原位形成Schiff碱进行,然后用苯甲酰溴/取代的苯甲酰溴进行碱介导的烷基化反应,最终导致分子内环化反应生成非对映异构体的混合物,具有非对映异构体选择性(高达dr = 99:1),分离为单一非对映异构体,产率中等至优异(41–92%)。此外,这种新的通用方法学为合成各种功能取代的苯并恶嗪支架提供了广阔的范围,并且可以进一步用作合成多面分子结构的基础,特别是在药物应用中。
更新日期:2020-06-19
中文翻译:

一锅多组分非对映选择性绿色合成3,4-二氢-2H-苯并[b] [1,4]恶嗪类似物。
已开发出一种新颖的绿色高效无催化剂,温和的单锅多组分合成策略,以构建取代的3,4-二氢-2 H-苯并[ b]] [1,4]恶嗪。该反应通过原位形成Schiff碱进行,然后用苯甲酰溴/取代的苯甲酰溴进行碱介导的烷基化反应,最终导致分子内环化反应生成非对映异构体的混合物,具有非对映异构体选择性(高达dr = 99:1),分离为单一非对映异构体,产率中等至优异(41–92%)。此外,这种新的通用方法学为合成各种功能取代的苯并恶嗪支架提供了广阔的范围,并且可以进一步用作合成多面分子结构的基础,特别是在药物应用中。






























 京公网安备 11010802027423号
京公网安备 11010802027423号