当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Dibenzoylbenzodipyrroles: Key Precursors for the Synthesis of Fused meso-Aryl Sapphyrins.
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2020-05-13 , DOI: 10.1021/acs.joc.0c00663 Prosenjit Isar 1 , Mangalampalli Ravikanth 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2020-05-13 , DOI: 10.1021/acs.joc.0c00663 Prosenjit Isar 1 , Mangalampalli Ravikanth 1
Affiliation
|
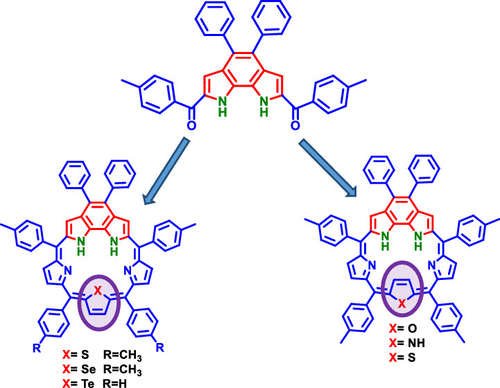
A simple strategy has been developed for the synthesis of α,α′-dibenzoylbenzodipyrroles, which are key synthons for the synthesis of fused porphyrinoids. α,α′-Dibenzoylbenzodipyrroles were characterized by high-resolution mass spectrometry (HRMS), NMR, and X-ray crystallography. To show the use of α,α′-dibenzoylbenzodipyrrole, we synthesized five different fused meso-aryl sapphyrins under acid-catalyzed reaction conditions. α,α′-Dibenzoylbenzodipyrrole was reduced to diol and condensed with five different tripyrranes such as aza, oxa, thia, selena, and telluratripyrranes under mild acid-catalyzed conditions to afford fused meso-aryl sapphyrins in 15–18% yields. One-dimensional (1D) and two-dimensional (2D) NMR studies revealed that in fused sapphyrins, the furan ring in oxabenzosapphyrin and the pyrrole ring in benzosapphyrin, which are present opposite to the benzodipyrrole moiety, attained ring inversion (inverted sapphyrins), whereas the selenophene ring in selenabenzosapphyrin and the tellurophene ring in tellurabenzosapphyrin did not show ring inversion (normal sapphyrins). However, thiabenzosapphyrin exhibits both normal and inverted conformations in different ratios. All fused sapphyrins showed typical aromatic absorption features; however, the absorption features of normal fused sapphyrins are different from the inverted fused sapphyrins. Redox studies indicate that normal fused sapphyrins are difficult to oxidize but easier to reduce compared to inverted fused sapphyrins. Density functional theory (DFT) studies support the experimental observations.
中文翻译:

二苯甲酰基苯并二吡咯:熔融中观芳基红木素合成的关键前体。
已经开发出一种简单的策略来合成α,α'-二苯甲酰基苯并二吡咯,这是合成稠合卟啉类化合物的关键合成子。α,α'-二苯甲酰基苯并二吡咯通过高分辨率质谱(HRMS),NMR和X射线晶体学表征。为了显示α,α′-二苯甲酰基苯并二吡咯的用途,我们在酸催化的反应条件下合成了五种不同的稠合的内消旋-芳基红景天苷。将α,α'-二苯甲酰苯并二吡咯还原为二醇,并在温和的酸催化条件下与五种不同的三吡喃,例如氮杂,氧杂,硫杂,硒,和三吡喃缩合,得到熔融的内消旋体芳基山ap苷的收率为15-18%。一维(1D)和二维(2D)NMR研究表明,在稠合的左旋卟啉中,与苯并二吡咯部分相对存在的氧杂苯并双卟啉中的呋喃环和苯并双卟啉中的吡咯环实现了环反转(反相的左旋卟啉),而硒代苯并二氮杂卟啉中的硒烯环和碲-苯并二氮杂卟啉中的碲二苯环未显示出环倒置(正常的番红素)。然而,硫代苯并尿素以不同的比率显示正常构象和反向构象。所有融合的鼠李素均表现出典型的芳香吸收特性;然而,正常的融合的鼠李素的吸收特征不同于倒置的融合的鼠李素。氧化还原研究表明,与倒置的融合的鼠李素相比,正常的融合的鼠李素难以氧化,但更易于还原。密度泛函理论(DFT)研究支持实验观察。
更新日期:2020-05-13
中文翻译:

二苯甲酰基苯并二吡咯:熔融中观芳基红木素合成的关键前体。
已经开发出一种简单的策略来合成α,α'-二苯甲酰基苯并二吡咯,这是合成稠合卟啉类化合物的关键合成子。α,α'-二苯甲酰基苯并二吡咯通过高分辨率质谱(HRMS),NMR和X射线晶体学表征。为了显示α,α′-二苯甲酰基苯并二吡咯的用途,我们在酸催化的反应条件下合成了五种不同的稠合的内消旋-芳基红景天苷。将α,α'-二苯甲酰苯并二吡咯还原为二醇,并在温和的酸催化条件下与五种不同的三吡喃,例如氮杂,氧杂,硫杂,硒,和三吡喃缩合,得到熔融的内消旋体芳基山ap苷的收率为15-18%。一维(1D)和二维(2D)NMR研究表明,在稠合的左旋卟啉中,与苯并二吡咯部分相对存在的氧杂苯并双卟啉中的呋喃环和苯并双卟啉中的吡咯环实现了环反转(反相的左旋卟啉),而硒代苯并二氮杂卟啉中的硒烯环和碲-苯并二氮杂卟啉中的碲二苯环未显示出环倒置(正常的番红素)。然而,硫代苯并尿素以不同的比率显示正常构象和反向构象。所有融合的鼠李素均表现出典型的芳香吸收特性;然而,正常的融合的鼠李素的吸收特征不同于倒置的融合的鼠李素。氧化还原研究表明,与倒置的融合的鼠李素相比,正常的融合的鼠李素难以氧化,但更易于还原。密度泛函理论(DFT)研究支持实验观察。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号