当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Photoinduced Palladium-Catalyzed Carbofunctionalization of Conjugated Dienes Proceeding via Radical-Polar Crossover Scenario: 1,2-Aminoalkylation and Beyond
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-05-14 , DOI: 10.1021/jacs.0c03993 Kelvin Pak Shing Cheung 1 , Daria Kurandina 1 , Tetsuji Yata 1 , Vladimir Gevorgyan 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-05-14 , DOI: 10.1021/jacs.0c03993 Kelvin Pak Shing Cheung 1 , Daria Kurandina 1 , Tetsuji Yata 1 , Vladimir Gevorgyan 1
Affiliation
|
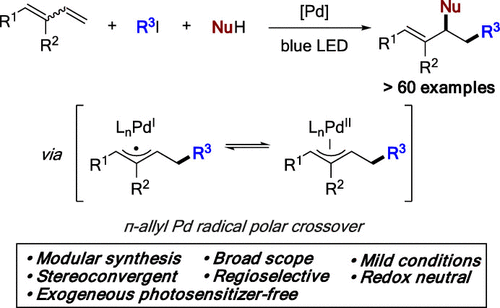
A photoinduced palladium-catalyzed 1,2-carbofunctionalization of conjugated dienes has been developed. This mild modular approach, which does not require employment of exogeneous photosensitizers and external oxidants, allows for efficient and highly regio- and ste-reoselective synthesis of a broad range of allylic amines from readily available 1,3-dienes, alkyl iodides, and amines. Employment of O- and C-nucleophiles toward oxyalkylation and dialkylation products was also demonstrated. A putative -allyl palladium radical-polar crossover path is proposed as a key event in this three-component coupling process. The utility of this protocol is highlighted by its appli-cation for derivatization of several amine-containing drugs.
中文翻译:

共轭二烯的光诱导钯催化碳官能化通过自由基-极性交叉场景进行:1,2-氨基烷基化及其他
已开发出光诱导钯催化的共轭二烯的 1,2-碳功能化。这种温和的模块化方法不需要使用外源光敏剂和外部氧化剂,允许从容易获得的 1,3-二烯、烷基碘和胺中高效且高度区域和立体选择性地合成各种烯丙胺. 还证明了使用 O-和 C-亲核试剂进行烷氧基化和二烷基化产物。推定的 π-烯丙基钯自由基 - 极性交叉路径被认为是该三组分耦合过程中的关键事件。该协议的实用性因其在几种含胺药物的衍生化中的应用而突出。
更新日期:2020-05-14
中文翻译:

共轭二烯的光诱导钯催化碳官能化通过自由基-极性交叉场景进行:1,2-氨基烷基化及其他
已开发出光诱导钯催化的共轭二烯的 1,2-碳功能化。这种温和的模块化方法不需要使用外源光敏剂和外部氧化剂,允许从容易获得的 1,3-二烯、烷基碘和胺中高效且高度区域和立体选择性地合成各种烯丙胺. 还证明了使用 O-和 C-亲核试剂进行烷氧基化和二烷基化产物。推定的 π-烯丙基钯自由基 - 极性交叉路径被认为是该三组分耦合过程中的关键事件。该协议的实用性因其在几种含胺药物的衍生化中的应用而突出。






























 京公网安备 11010802027423号
京公网安备 11010802027423号