当前位置:
X-MOL 学术
›
Adv. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
ROS and GSH Dual‐Responsive GEM Prodrug Micelles for ROS‐Triggered Fluorescence Turn on Bioimaging and Cancer Therapy
Advanced Materials Interfaces ( IF 4.3 ) Pub Date : 2020-05-12 , DOI: 10.1002/admi.202000294
Xin Su 1 , Weihua Zhuang 1 , Tao Yu 1 , Haiyang He 1 , Boxuan Ma 1 , Liang Chen 1 , Li Yang 1 , Gaocan Li 1 , Yunbing Wang 1
Advanced Materials Interfaces ( IF 4.3 ) Pub Date : 2020-05-12 , DOI: 10.1002/admi.202000294
Xin Su 1 , Weihua Zhuang 1 , Tao Yu 1 , Haiyang He 1 , Boxuan Ma 1 , Liang Chen 1 , Li Yang 1 , Gaocan Li 1 , Yunbing Wang 1
Affiliation
|
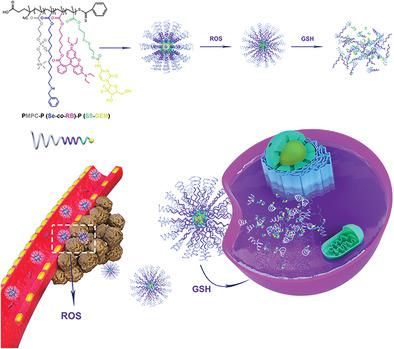
Nanocarriers with minimal drug leakage in blood and sufficient drug release at tumor sites are essential for tumor targeted drug delivery and bioimaging. Herein, a reactive oxygen species (ROS) and glutathione (GSH) dual‐responsive aggregation‐induced quenching (ACQ) prodrug micelle system is developed based on PMPC‐P (Se‐co‐RB)‐P (SS‐GEM) copolymer to avoid drug leakage and specifically locate the tumor via a ROS‐triggered fluorescence turn on manner. Selenium (Se) group is designed to respond to the ROS environment of tumor so as to turn on the fluorescence of Rhodamine B (RB) and Gemcitabine (GEM) is conjugated to the copolymer via a disulfide bond. These GEM prodrug micelles exhibit small particle size of 166.3 nm with uniformed size distribution, a very weak fluorescence emission, and minimal drug release with only 3.33% drug release under physiological conditions after 48 h. However, ROS (0.1% H2O2) and GSH (10 × 10−3 m) could synergistically promote disassembly of the micellar structure which causes accelerated drug release up to around 91.57% after 48 h. These prodrug micelles exhibit great in vitro and in vivo antitumor ability with minimal side effects. This novel drug delivery system provides new possibilities for designing stimuli‐responsive nanocarriers for tumor therapy and bioimaging.
中文翻译:

用于ROS触发荧光的ROS和GSH双反应GEM前药胶束开启了生物成像和癌症治疗的大门
具有最小的血液中药物泄漏和在肿瘤部位充分释放药物的纳米载体对于靶向肿瘤的药物递送和生物成像至关重要。在此,活性氧物质(ROS)和谷胱甘肽(GSH)的双响应聚集诱导的猝灭(ACQ)前药胶束系统是基于PMPC-P(SE-开发共‐RB)‐P(SS‐GEM)共聚物可避免药物泄漏,并通过ROS触发的荧光开启方式专门定位肿瘤。硒(Se)基团设计用于响应肿瘤的ROS环境,从而开启若丹明B(RB)的荧光,吉西他滨(GEM)通过二硫键与共聚物结合。这些GEM前药胶束在48小时后的生理条件下显示出166.3 nm的小粒径,具有均匀的粒径分布,非常弱的荧光发射和最小的药物释放,只有3.33%的药物释放。但是,ROS(0.1%H 2 O 2)和GSH(10×10 -3 m)可以协同促进胶束结构的分解,从而导致48小时后加速药物释放高达91.57%。这些前药胶束在体外和体内均具有出色的抗肿瘤能力,且副作用极小。这种新颖的药物输送系统为设计用于肿瘤治疗和生物成像的刺激响应性纳米载体提供了新的可能性。
更新日期:2020-05-12
中文翻译:

用于ROS触发荧光的ROS和GSH双反应GEM前药胶束开启了生物成像和癌症治疗的大门
具有最小的血液中药物泄漏和在肿瘤部位充分释放药物的纳米载体对于靶向肿瘤的药物递送和生物成像至关重要。在此,活性氧物质(ROS)和谷胱甘肽(GSH)的双响应聚集诱导的猝灭(ACQ)前药胶束系统是基于PMPC-P(SE-开发共‐RB)‐P(SS‐GEM)共聚物可避免药物泄漏,并通过ROS触发的荧光开启方式专门定位肿瘤。硒(Se)基团设计用于响应肿瘤的ROS环境,从而开启若丹明B(RB)的荧光,吉西他滨(GEM)通过二硫键与共聚物结合。这些GEM前药胶束在48小时后的生理条件下显示出166.3 nm的小粒径,具有均匀的粒径分布,非常弱的荧光发射和最小的药物释放,只有3.33%的药物释放。但是,ROS(0.1%H 2 O 2)和GSH(10×10 -3 m)可以协同促进胶束结构的分解,从而导致48小时后加速药物释放高达91.57%。这些前药胶束在体外和体内均具有出色的抗肿瘤能力,且副作用极小。这种新颖的药物输送系统为设计用于肿瘤治疗和生物成像的刺激响应性纳米载体提供了新的可能性。

































 京公网安备 11010802027423号
京公网安备 11010802027423号