当前位置:
X-MOL 学术
›
Solid State Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
One-pot hydrothermal preparation of Br-doped BiVO4 with enhanced visible-light photocatalytic activity
Solid State Sciences ( IF 3.4 ) Pub Date : 2020-07-01 , DOI: 10.1016/j.solidstatesciences.2020.106285 Chan Qin , Hongru Liao , Feiyang Rao , Junbo Zhong , Jianzhang Li
Solid State Sciences ( IF 3.4 ) Pub Date : 2020-07-01 , DOI: 10.1016/j.solidstatesciences.2020.106285 Chan Qin , Hongru Liao , Feiyang Rao , Junbo Zhong , Jianzhang Li
|
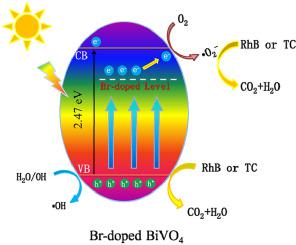
Abstract In this contribution, Br-doped BiVO4 photocatalysts were successfully obtained by a one-pot hydrothermal method. High resolution transmission electron microscopy (HRTEM) and X-ray photoelectron spectroscopy (XPS) were employed to confirm that Br was successfully doped into BiVO4. The separation performance of photo-generated carriers of Br-doped BiVO4 photocatalysts was studied by electrochemical technology and surface photovoltage spectroscopy (SPS). The results show that the existence of an impurity energy level formed by Br doping promotes the separation of photoinduced carriers. Photocatalytic properties of the samples towards degradation of Rhodamine B (RhB) and tetracycline (TC) were assessed under visible light irradiation. The experimental results reveal that photocatalytic performance of Br-doped BiVO4 photocatalysts is higher than that of the reference BiVO4. Under visible light irradiation, the abatement rate constant of RhB and TC over 3% Br-doped BiVO4 is 7 times of that over the reference BiVO4, respectively. The active free radicals were detected by electron spin resonance (ESR) and trapping experiments. The results indicate that superoxide free radical ( O2−) and hole (h+) are main active free species, and the 3% Br-doped BiVO4 produces more active free radicals. On account of all the observations, the separation and transfer mechanism of photogenerated charge was proposed. This work provides a useful reference for the preparation of highly efficient Br-doped photocatalytic materials.
中文翻译:

一锅水热法制备具有增强可见光催化活性的 Br 掺杂 BiVO4
摘要 在这项贡献中,通过一锅水热法成功地获得了 Br 掺杂的 BiVO4 光催化剂。采用高分辨率透射电子显微镜 (HRTEM) 和 X 射线光电子能谱 (XPS) 来确认 Br 成功掺杂到 BiVO4 中。采用电化学技术和表面光电压谱(SPS)研究了Br掺杂BiVO4光催化剂的光生载流子分离性能。结果表明,Br掺杂形成的杂质能级的存在促进了光生载流子的分离。在可见光照射下评估样品对罗丹明 B (RhB) 和四环素 (TC) 降解的光催化性能。实验结果表明,Br掺杂的BiVO4光催化剂的光催化性能高于参比BiVO4。在可见光照射下,RhB 和 TC 对 3% Br 掺杂的 BiVO4 的衰减速率常数分别是参考 BiVO4 的 7 倍。通过电子自旋共振(ESR)和捕获实验检测活性自由基。结果表明,超氧自由基(O2-)和空穴(h+)是主要的活性自由基,3% Br掺杂的BiVO4产生更多的活性自由基。综合以上观察,提出了光生电荷的分离和转移机制。该工作为制备高效的掺溴光催化材料提供了有益的参考。RhB 和 TC 对 3% Br 掺杂的 BiVO4 的减排速率常数分别是参考 BiVO4 的 7 倍。通过电子自旋共振(ESR)和捕获实验检测活性自由基。结果表明,超氧自由基(O2-)和空穴(h+)是主要的活性自由基,3% Br掺杂的BiVO4产生更多的活性自由基。综合以上观察,提出了光生电荷的分离和转移机制。该工作为制备高效的掺溴光催化材料提供了有益的参考。RhB 和 TC 对 3% Br 掺杂的 BiVO4 的减排速率常数分别是参考 BiVO4 的 7 倍。通过电子自旋共振(ESR)和捕获实验检测活性自由基。结果表明,超氧自由基(O2-)和空穴(h+)是主要的活性自由基,3% Br掺杂的BiVO4产生更多的活性自由基。综合以上观察,提出了光生电荷的分离和转移机制。该工作为制备高效的掺溴光催化材料提供了有益的参考。结果表明,超氧自由基(O2-)和空穴(h+)是主要的活性自由基,3% Br掺杂的BiVO4产生的活性自由基更多。综合以上观察,提出了光生电荷的分离和转移机制。该工作为制备高效的掺溴光催化材料提供了有益的参考。结果表明,超氧自由基(O2-)和空穴(h+)是主要的活性自由基,3% Br掺杂的BiVO4产生更多的活性自由基。综合以上观察,提出了光生电荷的分离和转移机制。该工作为制备高效的掺溴光催化材料提供了有益的参考。
更新日期:2020-07-01
中文翻译:

一锅水热法制备具有增强可见光催化活性的 Br 掺杂 BiVO4
摘要 在这项贡献中,通过一锅水热法成功地获得了 Br 掺杂的 BiVO4 光催化剂。采用高分辨率透射电子显微镜 (HRTEM) 和 X 射线光电子能谱 (XPS) 来确认 Br 成功掺杂到 BiVO4 中。采用电化学技术和表面光电压谱(SPS)研究了Br掺杂BiVO4光催化剂的光生载流子分离性能。结果表明,Br掺杂形成的杂质能级的存在促进了光生载流子的分离。在可见光照射下评估样品对罗丹明 B (RhB) 和四环素 (TC) 降解的光催化性能。实验结果表明,Br掺杂的BiVO4光催化剂的光催化性能高于参比BiVO4。在可见光照射下,RhB 和 TC 对 3% Br 掺杂的 BiVO4 的衰减速率常数分别是参考 BiVO4 的 7 倍。通过电子自旋共振(ESR)和捕获实验检测活性自由基。结果表明,超氧自由基(O2-)和空穴(h+)是主要的活性自由基,3% Br掺杂的BiVO4产生更多的活性自由基。综合以上观察,提出了光生电荷的分离和转移机制。该工作为制备高效的掺溴光催化材料提供了有益的参考。RhB 和 TC 对 3% Br 掺杂的 BiVO4 的减排速率常数分别是参考 BiVO4 的 7 倍。通过电子自旋共振(ESR)和捕获实验检测活性自由基。结果表明,超氧自由基(O2-)和空穴(h+)是主要的活性自由基,3% Br掺杂的BiVO4产生更多的活性自由基。综合以上观察,提出了光生电荷的分离和转移机制。该工作为制备高效的掺溴光催化材料提供了有益的参考。RhB 和 TC 对 3% Br 掺杂的 BiVO4 的减排速率常数分别是参考 BiVO4 的 7 倍。通过电子自旋共振(ESR)和捕获实验检测活性自由基。结果表明,超氧自由基(O2-)和空穴(h+)是主要的活性自由基,3% Br掺杂的BiVO4产生更多的活性自由基。综合以上观察,提出了光生电荷的分离和转移机制。该工作为制备高效的掺溴光催化材料提供了有益的参考。结果表明,超氧自由基(O2-)和空穴(h+)是主要的活性自由基,3% Br掺杂的BiVO4产生的活性自由基更多。综合以上观察,提出了光生电荷的分离和转移机制。该工作为制备高效的掺溴光催化材料提供了有益的参考。结果表明,超氧自由基(O2-)和空穴(h+)是主要的活性自由基,3% Br掺杂的BiVO4产生更多的活性自由基。综合以上观察,提出了光生电荷的分离和转移机制。该工作为制备高效的掺溴光催化材料提供了有益的参考。

































 京公网安备 11010802027423号
京公网安备 11010802027423号