当前位置:
X-MOL 学术
›
ACS Appl. Energy Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Significant Reduction in the Interfacial Resistance of Garnet-Type Solid Electrolyte and Lithium Metal by a Thick Amorphous Lithium Silicate Layer
ACS Applied Energy Materials ( IF 5.4 ) Pub Date : 2020-05-13 00:00:00 , DOI: 10.1021/acsaem.0c00511 Nataly Carolina Rosero-Navarro 1 , Ryunosuke Kajiura 2 , Randy Jalem 3, 4, 5, 6 , Yoshitaka Tateyama 3, 4, 6 , Akira Miura 1 , Kiyoharu Tadanaga 1
ACS Applied Energy Materials ( IF 5.4 ) Pub Date : 2020-05-13 00:00:00 , DOI: 10.1021/acsaem.0c00511 Nataly Carolina Rosero-Navarro 1 , Ryunosuke Kajiura 2 , Randy Jalem 3, 4, 5, 6 , Yoshitaka Tateyama 3, 4, 6 , Akira Miura 1 , Kiyoharu Tadanaga 1
Affiliation
|
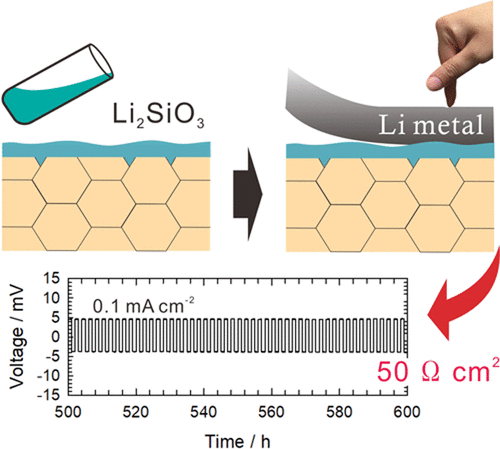
The poor ionic and electronic contacts between electrodes and the electrolyte is a critical problem in the fabrication of all-solid-state lithium batteries based on garnet-type oxide solid electrolytes. The interfacial resistance with lithium metal anode has been widely studied because the use of lithium metal anodes can provide energy storage systems with the largest specific energy. Here, an amorphous lithium silicate interlayer is proposed to obtain a low interfacial resistance of the garnet solid electrolyte with lithium metal. The lithium silicate layer is deposited on the garnet solid electrolyte by a liquid-phase process followed by heat treatment, and metallic lithium is put on the modified surface by just pressing, without heat treatment. This approach achieves a low interfacial resistance of 44 Ω cm2 and a stable dissolution/deposition cycling behavior at a current density of up to 0.5 mA cm–2. A quasi-solid-state battery constructed with the garnet solid electrolyte modified with the lithium silicate layer, lithium metal, and LiNi1/3Co1/3Mn1/3O2 electrode shows a stable cycling performance at 25 °C (124 mAh g–1 after 100 cycles). Thermodynamic calculations suggest that the effective contact between lithium metal and lithium silicate is created by the formation of Li–Si phases at the interface.
中文翻译:

厚的非晶硅酸锂层显着降低了石榴石型固体电解质和锂金属的界面电阻
在基于石榴石型氧化物固体电解质的全固态锂电池的制造中,电极与电解质之间不良的离子和电子接触是关键问题。与锂金属阳极的界面电阻已得到广泛研究,因为使用锂金属阳极可以为储能系统提供最大的比能。在此,提出了非晶硅酸锂夹层,以使石榴石固体电解质与锂金属的界面电阻降低。硅酸锂层通过液相工艺随后进行热处理而沉积在石榴石固体电解质上,并且金属锂仅通过加压而无需热处理而被置于改性表面上。这种方法实现了44Ωcm 2的低界面电阻在电流密度高达0.5 mA cm –2时具有稳定的溶解/沉积循环行为。由石榴石固体电解质修饰的硅酸锂层,锂金属和LiNi 1/3 Co 1/3 Mn 1/3 O 2电极构成的准固态电池在25°C时具有稳定的循环性能(124 mAh g –1(100次循环后)。热力学计算表明,锂金属和硅酸锂之间的有效接触是通过在界面处形成Li-Si相来实现的。
更新日期:2020-05-13
中文翻译:

厚的非晶硅酸锂层显着降低了石榴石型固体电解质和锂金属的界面电阻
在基于石榴石型氧化物固体电解质的全固态锂电池的制造中,电极与电解质之间不良的离子和电子接触是关键问题。与锂金属阳极的界面电阻已得到广泛研究,因为使用锂金属阳极可以为储能系统提供最大的比能。在此,提出了非晶硅酸锂夹层,以使石榴石固体电解质与锂金属的界面电阻降低。硅酸锂层通过液相工艺随后进行热处理而沉积在石榴石固体电解质上,并且金属锂仅通过加压而无需热处理而被置于改性表面上。这种方法实现了44Ωcm 2的低界面电阻在电流密度高达0.5 mA cm –2时具有稳定的溶解/沉积循环行为。由石榴石固体电解质修饰的硅酸锂层,锂金属和LiNi 1/3 Co 1/3 Mn 1/3 O 2电极构成的准固态电池在25°C时具有稳定的循环性能(124 mAh g –1(100次循环后)。热力学计算表明,锂金属和硅酸锂之间的有效接触是通过在界面处形成Li-Si相来实现的。

































 京公网安备 11010802027423号
京公网安备 11010802027423号