当前位置:
X-MOL 学术
›
ChemistrySelect
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Tetrazole and Azido Derivatives of Pyrimidine: Synthesis, Mechanism, Thermal Behaviour & Steering of Azido–Tetrazole Equilibrium
ChemistrySelect ( IF 1.9 ) Pub Date : 2020-05-12 , DOI: 10.1002/slct.202001087
Saira Manzoor 1 , Jun‐Qing Yang 1 , Qamar‐un‐nisa Tariq 1 , Hao‐Zheng Mei 1 , Zhen‐Li Yang 1 , Yong Hu 1 , Wen‐Li Cao 1 , Valery P. Sinditskii 2 , Jian‐Guo Zhang 1
ChemistrySelect ( IF 1.9 ) Pub Date : 2020-05-12 , DOI: 10.1002/slct.202001087
Saira Manzoor 1 , Jun‐Qing Yang 1 , Qamar‐un‐nisa Tariq 1 , Hao‐Zheng Mei 1 , Zhen‐Li Yang 1 , Yong Hu 1 , Wen‐Li Cao 1 , Valery P. Sinditskii 2 , Jian‐Guo Zhang 1
Affiliation
|
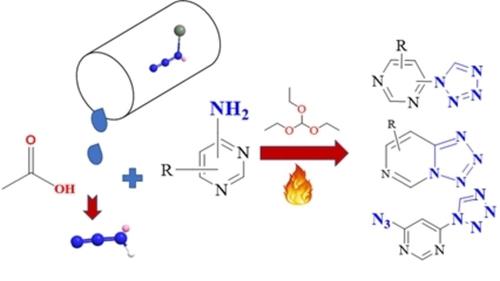
A new family of pyrimidine modified tetrazole & azido derivatives (1‐16) was developed using the conventional and nucleophilic substitution methods. The 1H‐tetrazol‐1‐yl)pyrimidine (1‐10) compounds were prepared via traditional cycloaddition and condensation method. The compounds tetrazolo[1,5‐a]pyrimidine (11 a,12 a, and 13 a), azido‐(1H‐tetrazol‐1‐yl)pyrimidine (14 a and 15 a) and tetrazolo[1,5‐c]pyrimidine (16 a) were synthesized by simultaneously introducing conventional and nucleophilic substitution approaches. The latter technique was easy to process and reduce the synthesis time. The factors (solvent, temperature, steric effect, electron‐donating groups, and electron‐withdrawing groups) were found responsible for steering the azido‐tetrazole equilibrium in the compounds 11 a, 12 a, 13 a, and 16 a. All the prepared compounds were well characterized including single‐crystal X‐ray diffraction structures of 1, 3, 4, 6, 8, 10, 11 a, 12 a, 13 a, 14 a, 15 a, and 16 a. Thermal behaviour was investigated by differential scanning calorimetry (DSC) and thermal gravimetric analysis (TGA). The current work is significant to the development of a new class of pyrimidine modified tetrazole and azido derivatives in sense of easy reaction approach, good to excellent yields, safe process, and simple work‐ups.
中文翻译:

嘧啶的四唑和叠氮基衍生物:叠氮基-四唑平衡的合成,机理,热行为和操纵
使用常规和亲核取代方法开发了一个新的嘧啶修饰的四唑和叠氮基衍生物家族(1-16)。1H-四唑-1-基)嘧啶(1-10)化合物是通过传统的环加成和缩合方法制备的。化合物tetrazolo [1,5-a]嘧啶(11 a,12 a和13 a),叠氮基(1H-tetrazol-1-yl)嘧啶(14 a和15 a)和tetrazolo [1,5- c嘧啶(16 a)是通过同时引入常规和亲核取代方法合成的。后一种技术易于加工并减少了合成时间。的因素(溶剂,温度,空间位阻效应,给电子基团,和吸电子基团)被发现负责化合物中操纵叠氮基四唑平衡11,12,13,和16。所有制备的化合物进行充分表征,包括单晶X射线衍射结构1,3,4,6,8,10,11,12上的,13a,14a,15a和16a。通过差示扫描量热法(DSC)和热重分析(TGA)研究了热行为。从简便的反应方法,良好的产率到优良的方法,安全的方法和简单的后处理的意义上讲,当前的工作对于开发新型的嘧啶改性的四唑和叠氮基衍生物具有重要意义。
更新日期:2020-05-12
中文翻译:

嘧啶的四唑和叠氮基衍生物:叠氮基-四唑平衡的合成,机理,热行为和操纵
使用常规和亲核取代方法开发了一个新的嘧啶修饰的四唑和叠氮基衍生物家族(1-16)。1H-四唑-1-基)嘧啶(1-10)化合物是通过传统的环加成和缩合方法制备的。化合物tetrazolo [1,5-a]嘧啶(11 a,12 a和13 a),叠氮基(1H-tetrazol-1-yl)嘧啶(14 a和15 a)和tetrazolo [1,5- c嘧啶(16 a)是通过同时引入常规和亲核取代方法合成的。后一种技术易于加工并减少了合成时间。的因素(溶剂,温度,空间位阻效应,给电子基团,和吸电子基团)被发现负责化合物中操纵叠氮基四唑平衡11,12,13,和16。所有制备的化合物进行充分表征,包括单晶X射线衍射结构1,3,4,6,8,10,11,12上的,13a,14a,15a和16a。通过差示扫描量热法(DSC)和热重分析(TGA)研究了热行为。从简便的反应方法,良好的产率到优良的方法,安全的方法和简单的后处理的意义上讲,当前的工作对于开发新型的嘧啶改性的四唑和叠氮基衍生物具有重要意义。

































 京公网安备 11010802027423号
京公网安备 11010802027423号