当前位置:
X-MOL 学术
›
Chem. Eur. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
On the Limited Stability of BDPA Radicals.
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2020-05-12 , DOI: 10.1002/chem.202001084 Sucharita Mandal 1 , Snorri Th Sigurdsson 1
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2020-05-12 , DOI: 10.1002/chem.202001084 Sucharita Mandal 1 , Snorri Th Sigurdsson 1
Affiliation
|
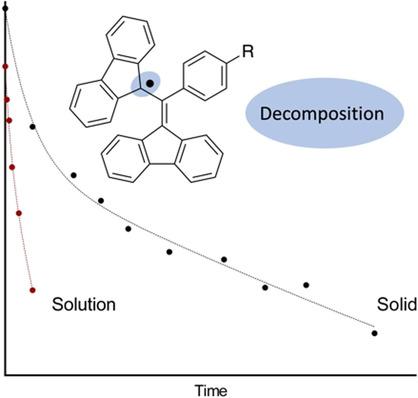
1,3‐Bis(diphenylene)‐2‐phenylallyl (BDPA)‐based radicals are of interest as polarizing agents for dynamic nuclear polarization (DNP). For this purpose, a BDPA‐nitroxide biradical, employing a phosphodiester linkage, was synthesized. Contrary to what is commonly assumed, BDPA‐derived radicals were observed to have limited stability. Hence, the effects of various factors on the stability of BDPA radicals were investigated. Solvent polarity was found to play a significant role on degradation; a polar BDPA radical was observed to degrade faster in a non‐polar solvent, whereas non‐polar radicals were more unstable in polar solvents. The rate of decomposition was found to increase non‐linearly with increasing radical concentration; a 2‐fold increase in concentration led to a 3‐fold increase in the rate of degradation. Collectively, these results indicate that the dimerization is a significant degradation pathway for BDPA radicals and indeed, a dimer of one BDPA radical was detected by mass spectrometry.
中文翻译:

关于BDPA自由基的有限稳定性。
基于1,3-双(二亚苯基)-2-苯基烯丙基(BDPA)的自由基作为动态核极化(DNP)的极化剂受到关注。为此目的,采用磷酸二酯键合成了BDPA-硝基氧双自由基。与通常的假设相反,观察到BDPA衍生的自由基具有有限的稳定性。因此,研究了各种因素对BDPA自由基稳定性的影响。发现溶剂极性在降解中起重要作用。观察到极性BDPA自由基在非极性溶剂中降解更快,而非极性自由基在极性溶剂中更不稳定。发现分解速率随自由基浓度的增加而非线性增加;浓度增加2倍,降解速率增加3倍。总的来说,
更新日期:2020-05-12
中文翻译:

关于BDPA自由基的有限稳定性。
基于1,3-双(二亚苯基)-2-苯基烯丙基(BDPA)的自由基作为动态核极化(DNP)的极化剂受到关注。为此目的,采用磷酸二酯键合成了BDPA-硝基氧双自由基。与通常的假设相反,观察到BDPA衍生的自由基具有有限的稳定性。因此,研究了各种因素对BDPA自由基稳定性的影响。发现溶剂极性在降解中起重要作用。观察到极性BDPA自由基在非极性溶剂中降解更快,而非极性自由基在极性溶剂中更不稳定。发现分解速率随自由基浓度的增加而非线性增加;浓度增加2倍,降解速率增加3倍。总的来说,































 京公网安备 11010802027423号
京公网安备 11010802027423号