当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Catalytic Aerobic Cross-Dehydrogenative Coupling of Azlactones en Route to α,α-Disubstituted α-Amino Acids.
Organic Letters ( IF 4.9 ) Pub Date : 2020-05-12 , DOI: 10.1021/acs.orglett.0c01248
Taro Tsuji 1 , Takafumi Tanaka 1 , Tsukushi Tanaka 1 , Ryo Yazaki 1 , Takashi Ohshima 1
Organic Letters ( IF 4.9 ) Pub Date : 2020-05-12 , DOI: 10.1021/acs.orglett.0c01248
Taro Tsuji 1 , Takafumi Tanaka 1 , Tsukushi Tanaka 1 , Ryo Yazaki 1 , Takashi Ohshima 1
Affiliation
|
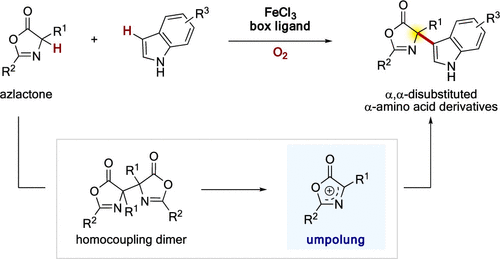
We developed a catalytic aerobic method to synthesize α,α-disubstituted α-amino acids through cross-dehydrogenative coupling of azlactones. Combining an iron catalyst with a bisoxazolidine ligand resulted in high catalytic performance, and cross-coupling with an indole proceeded smoothly under aerobic conditions. A wide variety of α-aryl and aliphatic amino acid derived azlactones were applied to the present catalysis. In addition, a quaternary carbon could be constructed using oxindole and benzofuranone under aerobic conditions.
中文翻译:

氮杂内酯在有氧催化的交叉脱氢偶联过程中生成α,α-二取代的α-氨基酸。
我们开发了一种催化好氧方法,可通过氮杂内酯的交叉脱氢偶联来合成α,α-二取代的α-氨基酸。将铁催化剂与双恶唑烷配体结合使用可获得较高的催化性能,在有氧条件下,与吲哚的交叉偶联反应顺利进行。多种由α-芳基和脂族氨基酸衍生的z内酯被用于本催化。此外,在有氧条件下,可以使用羟吲哚和苯并呋喃酮构建季碳。
更新日期:2020-05-12
中文翻译:

氮杂内酯在有氧催化的交叉脱氢偶联过程中生成α,α-二取代的α-氨基酸。
我们开发了一种催化好氧方法,可通过氮杂内酯的交叉脱氢偶联来合成α,α-二取代的α-氨基酸。将铁催化剂与双恶唑烷配体结合使用可获得较高的催化性能,在有氧条件下,与吲哚的交叉偶联反应顺利进行。多种由α-芳基和脂族氨基酸衍生的z内酯被用于本催化。此外,在有氧条件下,可以使用羟吲哚和苯并呋喃酮构建季碳。































 京公网安备 11010802027423号
京公网安备 11010802027423号