当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Luminescence Onset and Mechanism of the Formation of Gold(I)–Thiolate Complexes as the Precursors to Nanoparticles
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2020-05-11 , DOI: 10.1021/acs.jpcc.0c02725 Gabriel A Palermo 1 , Mehnaz Tarannum 1 , Shunji Egusa 1
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2020-05-11 , DOI: 10.1021/acs.jpcc.0c02725 Gabriel A Palermo 1 , Mehnaz Tarannum 1 , Shunji Egusa 1
Affiliation
|
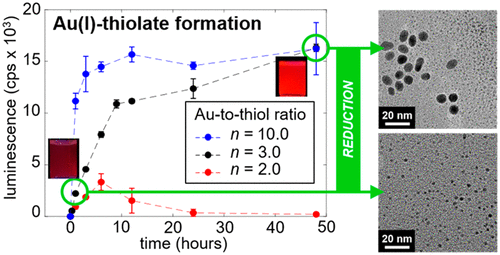
Gold(I) (Au(I))–thiolate complexes are widely believed as the precursors to Au nanoparticle formations. While the literature suggests that the Au(III)-to-thiol ligand stoichiometric ratio of 1:3 is required to reduce a Au(III) and yield a Au(I)–thiolate, other stoichiometric ratios are also known to produce Au nanoparticles upon reduction. Using the characteristic red luminescence of Au(I)-alkanethiolates, we examined the process of their formations and their implications on the Au nanoparticle synthesis in detail. The onset of the luminescence, correlated with the Au(I)–thiolate formation, as well as the kinetics of the luminophore formation were evaluated in terms of the Au(III)-to-alkanethiol ratios. The onset of the luminescence was affected significantly by the solvent polarity during reaction but not post reaction. We found that the kinetics of the luminophore formation can vary widely, requiring from minutes to 24 h for completion depending on the thiol ligands and molar ratios, as well as solvents. This information could help in designing Au nanoparticle syntheses with the logical choice of Au(III)-to-thiol ratio, solvent, and the timing of reduction.
中文翻译:

作为纳米粒子前体的金(I)-硫醇盐配合物的发光开始和形成机制
金(I)(Au(I))-硫醇盐复合物被广泛认为是金纳米颗粒形成的前体。虽然文献表明需要 1:3 的 Au(III)-硫醇配体化学计量比来还原 Au(III) 并产生 Au(I)-硫醇盐,但也已知其他化学计量比可产生 Au 纳米颗粒减少时。使用 Au(I)-alkanethiolates 的特征红色发光,我们详细检查了它们的形成过程及其对 Au 纳米颗粒合成的影响。发光的开始,与 Au(I)-硫醇盐的形成相关,以及发光团形成的动力学是根据 Au(III)-链烷硫醇的比率来评估的。发光的开始受反应期间溶剂极性的显着影响,但不受反应后的影响。我们发现发光团形成的动力学变化很大,需要几分钟到 24 小时才能完成,具体取决于硫醇配体和摩尔比,以及溶剂。这些信息可以帮助设计 Au 纳米颗粒合成,并合理选择 Au (III) 与硫醇的比例、溶剂和还原时间。
更新日期:2020-05-11
中文翻译:

作为纳米粒子前体的金(I)-硫醇盐配合物的发光开始和形成机制
金(I)(Au(I))-硫醇盐复合物被广泛认为是金纳米颗粒形成的前体。虽然文献表明需要 1:3 的 Au(III)-硫醇配体化学计量比来还原 Au(III) 并产生 Au(I)-硫醇盐,但也已知其他化学计量比可产生 Au 纳米颗粒减少时。使用 Au(I)-alkanethiolates 的特征红色发光,我们详细检查了它们的形成过程及其对 Au 纳米颗粒合成的影响。发光的开始,与 Au(I)-硫醇盐的形成相关,以及发光团形成的动力学是根据 Au(III)-链烷硫醇的比率来评估的。发光的开始受反应期间溶剂极性的显着影响,但不受反应后的影响。我们发现发光团形成的动力学变化很大,需要几分钟到 24 小时才能完成,具体取决于硫醇配体和摩尔比,以及溶剂。这些信息可以帮助设计 Au 纳米颗粒合成,并合理选择 Au (III) 与硫醇的比例、溶剂和还原时间。































 京公网安备 11010802027423号
京公网安备 11010802027423号