当前位置:
X-MOL 学术
›
Sci. Total Environ.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enhanced decomposition of H2O2 by molybdenum disulfide in a Fenton-like process for abatement of organic micropollutants.
Science of the Total Environment ( IF 8.2 ) Pub Date : 2020-05-11 , DOI: 10.1016/j.scitotenv.2020.139335 Hongwei Luo 1 , Ying Cheng 1 , Yifeng Zeng 1 , Kai Luo 1 , Xiangliang Pan 1
Science of the Total Environment ( IF 8.2 ) Pub Date : 2020-05-11 , DOI: 10.1016/j.scitotenv.2020.139335 Hongwei Luo 1 , Ying Cheng 1 , Yifeng Zeng 1 , Kai Luo 1 , Xiangliang Pan 1
Affiliation
|
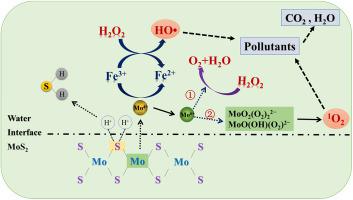
Accelerating the rate-limiting step of Fe3+/Fe2+ conversion is a major challenge for H2O2 decomposition in conventional Fenton process. In this study, the catalytic mechanism of H2O2 by molybdenum disulfide (MoS2) nanoparticles and Fe3+ ions was revealed and the abatement of organic micropollutants was investigated. The presence of both MoS2 and Fe3+ can efficiently decompose H2O2. Reaction system of H2O2/MoS2/Fe3+ is found to remove most of the tested pollutants by over 80% (except 65.9% for carbamazepine) within 60 min at pH of 3.0. Effective pH range of this reaction system can be extended to pH of 5.0. Adding MoS2 to Fe3+/H2O2 system promotes the Fe3+/Fe2+ cycle and improves the reaction rate between Fe3+ and H2O2. The formation of Mo6+ ions and Mo6+ peroxo-complexes is beneficial to H2O2 decomposition and pollutant degradation. Electron paramagnetic resonance (EPR) measurements and quenching experiments confirm the important role of hydroxyl radicals in H2O2/MoS2/Fe3+ system. Chloride ions (Cl-) promote degradation, while bicarbonate ions (HCO3-) inhibit degradation. As H2O2 concentration increases from nil to 1.0 mM, the value of total EE/O decreases from 0.083 to 0.003 kWh L-1, and the most energy efficient condition is determined. This study provides a new pathway for efficient decomposition of H2O2 by Fe3+ ions in an extended pH range, which is considered a facile and promising strategy for wastewater treatment.
中文翻译:

在Fenton样工艺中通过二硫化钼增强H2O2的分解,以减少有机微量污染物。
加速Fe3 + / Fe2 +转化的限速步骤是常规Fenton工艺中H2O2分解的主要挑战。在这项研究中,揭示了二硫化钼(MoS2)纳米颗粒和Fe3 +离子对H2O2的催化机理,并研究了有机微量污染物的消除。MoS2和Fe3 +的存在可以有效地分解H2O2。发现H2O2 / MoS2 / Fe3 +的反应系统在pH值为3.0的情况下,在60分钟内可将大多数测试污染物去除80%以上(卡马西平除外65.9%)。该反应系统的有效pH范围可以扩展到5.0。在Fe3 + / H2O2体系中加入MoS2可以促进Fe3 + / Fe2 +循环,并提高Fe3 +与H2O2之间的反应速率。Mo6 +离子和Mo6 +过氧配合物的形成有利于H2O2分解和污染物降解。电子顺磁共振(EPR)测量和淬灭实验证实了羟基自由基在H2O2 / MoS2 / Fe3 +系统中的重要作用。氯离子(Cl-)促进降解,而碳酸氢根离子(HCO3-)抑制降解。随着H2O2浓度从零增加到1.0 mM,总EE / O值从0.083减少到0.003 kWh L-1,并确定了最节能的条件。这项研究提供了一个新的途径,可以在扩展的pH范围内通过Fe3 +离子有效分解H2O2,这被认为是一种可行的废水处理策略。随着H2O2浓度从零增加到1.0 mM,总EE / O值从0.083减少到0.003 kWh L-1,并确定了最节能的条件。这项研究提供了一个新的途径,可以在扩展的pH范围内通过Fe3 +离子有效分解H2O2,这被认为是一种可行的废水处理策略。随着H2O2浓度从零增加到1.0 mM,总EE / O值从0.083减少到0.003 kWh L-1,并确定了最节能的条件。这项研究提供了一个新的途径,可以在扩展的pH范围内通过Fe3 +离子有效分解H2O2,这被认为是一种可行的废水处理策略。
更新日期:2020-05-11
中文翻译:

在Fenton样工艺中通过二硫化钼增强H2O2的分解,以减少有机微量污染物。
加速Fe3 + / Fe2 +转化的限速步骤是常规Fenton工艺中H2O2分解的主要挑战。在这项研究中,揭示了二硫化钼(MoS2)纳米颗粒和Fe3 +离子对H2O2的催化机理,并研究了有机微量污染物的消除。MoS2和Fe3 +的存在可以有效地分解H2O2。发现H2O2 / MoS2 / Fe3 +的反应系统在pH值为3.0的情况下,在60分钟内可将大多数测试污染物去除80%以上(卡马西平除外65.9%)。该反应系统的有效pH范围可以扩展到5.0。在Fe3 + / H2O2体系中加入MoS2可以促进Fe3 + / Fe2 +循环,并提高Fe3 +与H2O2之间的反应速率。Mo6 +离子和Mo6 +过氧配合物的形成有利于H2O2分解和污染物降解。电子顺磁共振(EPR)测量和淬灭实验证实了羟基自由基在H2O2 / MoS2 / Fe3 +系统中的重要作用。氯离子(Cl-)促进降解,而碳酸氢根离子(HCO3-)抑制降解。随着H2O2浓度从零增加到1.0 mM,总EE / O值从0.083减少到0.003 kWh L-1,并确定了最节能的条件。这项研究提供了一个新的途径,可以在扩展的pH范围内通过Fe3 +离子有效分解H2O2,这被认为是一种可行的废水处理策略。随着H2O2浓度从零增加到1.0 mM,总EE / O值从0.083减少到0.003 kWh L-1,并确定了最节能的条件。这项研究提供了一个新的途径,可以在扩展的pH范围内通过Fe3 +离子有效分解H2O2,这被认为是一种可行的废水处理策略。随着H2O2浓度从零增加到1.0 mM,总EE / O值从0.083减少到0.003 kWh L-1,并确定了最节能的条件。这项研究提供了一个新的途径,可以在扩展的pH范围内通过Fe3 +离子有效分解H2O2,这被认为是一种可行的废水处理策略。































 京公网安备 11010802027423号
京公网安备 11010802027423号