Molecular Cell ( IF 14.5 ) Pub Date : 2020-05-11 , DOI: 10.1016/j.molcel.2020.04.019
Sang-Ah Kim 1 , Jiang Zhu 1 , Neela Yennawar 2 , Priit Eek 1 , Song Tan 1
|
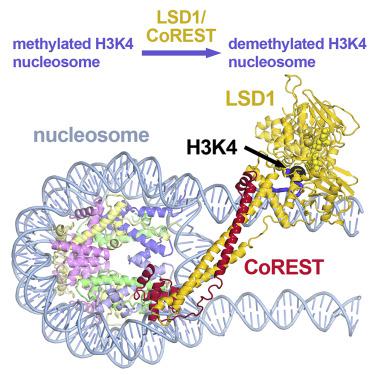
LSD1 (lysine specific demethylase; also known as KDM1A), the first histone demethylase discovered, regulates cell-fate determination and is overexpressed in multiple cancers. LSD1 demethylates histone H3 Lys4, an epigenetic mark for active genes, but requires the CoREST repressor to act on nucleosome substrates. To understand how an accessory subunit (CoREST) enables a chromatin enzyme (LSD1) to function on a nucleosome and not just histones, we have determined the crystal structure of the LSD1/CoREST complex bound to a 191-bp nucleosome. We find that the LSD1 catalytic domain binds extranucleosomal DNA and is unexpectedly positioned 100 Å away from the nucleosome core. CoREST makes critical contacts with both histone and DNA components of the nucleosome, explaining its essential function in demethylating nucleosome substrates. Our studies also show that the LSD1(K661A) frequently used as a catalytically inactive mutant in vivo (based on in vitro peptide studies) actually retains substantial H3K4 demethylase activity on nucleosome substrates.
中文翻译:

LSD1 / CoREST组蛋白去甲基化酶的晶体结构绑定到其核小体底物。
LSD1(赖氨酸特异性脱甲基酶;也称为KDM1A)是第一个发现的组蛋白脱甲基酶,它调节细胞命运的确定,并在多种癌症中过表达。LSD1使组蛋白H3 Lys4脱甲基,组蛋白H3 Lys4是活性基因的表观遗传标记,但需要CoREST阻遏物才能作用于核小体底物。为了了解辅助亚基(CoREST)如何使染色质酶(LSD1)不仅在组蛋白上在核小体上起作用,我们确定了与191 bp核小体结合的LSD1 / CoREST复合物的晶体结构。我们发现,LSD1催化结构域结合核外体DNA,并意外地位于离核小体核心100Å的位置。CoREST与核小体的组蛋白和DNA组分都进行了关键接触,从而解释了其在使核小体底物脱甲基化中的基本功能。体内(基于体外肽研究)实际上在核小体底物上保留了大量的H3K4脱甲基酶活性。

































 京公网安备 11010802027423号
京公网安备 11010802027423号