当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of 2-Imino-1,3,4-thiadiazoles from Hydrazides and Isothiocyanates via Sequential Oxidation and P(NMe2)3-Mediated Annulation Reactions.
Organic Letters ( IF 4.9 ) Pub Date : 2020-05-10 , DOI: 10.1021/acs.orglett.0c01393 Zhengyan Huang 1 , Qianqian Zhang 1 , Qiongli Zhao 1 , Wenquan Yu 1 , Junbiao Chang 1
Organic Letters ( IF 4.9 ) Pub Date : 2020-05-10 , DOI: 10.1021/acs.orglett.0c01393 Zhengyan Huang 1 , Qianqian Zhang 1 , Qiongli Zhao 1 , Wenquan Yu 1 , Junbiao Chang 1
Affiliation
|
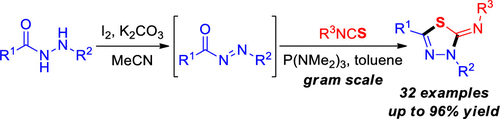
A P(NMe2)3-mediated annulation reaction of N-acyldiazenes with isothiocyanates, producing 2-imino-1,3,4-thiadiazoles, is reported. This reaction proceeds well with crude N-acyldiazenes derived from the oxidation of hydrazides by iodine and permits the sequential synthesis of products directly from hydrazides without purification of the less stable N-acyldiazene intermediates. The reaction does not require transition metals and is a simple, scalable operation with broad substrate scope.
中文翻译:

由酰肼和异硫氰酸酯通过顺序氧化和P(NMe2)3-介导的环化反应合成2-Imino-1,3,4-噻二唑。
报道了AP(NMe 2)3介导的N-酰基二氮烯与异硫氰酸酯的环化反应,产生2-亚氨基-1,3,4-噻二唑。该反应与衍生自酰肼被碘氧化的粗制N-酰基二氮烯进行得很好,并允许直接从酰肼连续合成产物,而无需纯化不稳定的N-酰基二氮烯中间体。该反应不需要过渡金属,是一种简单,可扩展的操作,具有广泛的底物范围。
更新日期:2020-05-10
中文翻译:

由酰肼和异硫氰酸酯通过顺序氧化和P(NMe2)3-介导的环化反应合成2-Imino-1,3,4-噻二唑。
报道了AP(NMe 2)3介导的N-酰基二氮烯与异硫氰酸酯的环化反应,产生2-亚氨基-1,3,4-噻二唑。该反应与衍生自酰肼被碘氧化的粗制N-酰基二氮烯进行得很好,并允许直接从酰肼连续合成产物,而无需纯化不稳定的N-酰基二氮烯中间体。该反应不需要过渡金属,是一种简单,可扩展的操作,具有广泛的底物范围。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号