当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The Origin of Capacity Fade in the Li2MnO3·LiMO2(M= Li, Ni, Co, Mn) Microsphere Positive Electrode: AnOperandoNeutron Diffraction and Transmission X-ray Microscopy Study
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2016-07-06 , DOI: 10.1021/jacs.6b03932 Chih-Jung Chen, Wei Kong Pang, Tatsuhiro Mori, Vanessa K. Peterson, Neeraj Sharma, Po-Han Lee, She-huang Wu, Chun-Chieh Wang, Yen-Fang Song, Ru-Shi Liu
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2016-07-06 , DOI: 10.1021/jacs.6b03932 Chih-Jung Chen, Wei Kong Pang, Tatsuhiro Mori, Vanessa K. Peterson, Neeraj Sharma, Po-Han Lee, She-huang Wu, Chun-Chieh Wang, Yen-Fang Song, Ru-Shi Liu
|
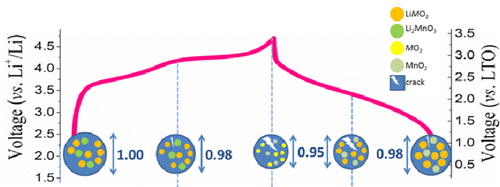
The mechanism of capacity fade of the Li2MnO3·LiMO2 (M = Li, Ni, Co, Mn) composite positive electrode within a full cell was investigated using a combination of operando neutron powder diffraction and transmission X-ray microscopy methods, enabling the phase, crystallographic, and morphological evolution of the material during electrochemical cycling to be understood. The electrode was shown to initially consist of 73(1) wt % R3̅m LiMO2 with the remaining 27(1) wt % C2/m Li2MnO3 likely existing as an intergrowth. Cracking in the Li2MnO3·LiMO2 electrode particle under operando microscopy observation was revealed to be initiated by the solid-solution reaction of the LiMO2 phase on charge to 4.55 V vs Li(+)/Li and intensified during further charge to 4.7 V vs Li(+)/Li during the concurrent two-phase reaction of the LiMO2 phase, involving the largest lattice change of any phase, and oxygen evolution from the Li2MnO3 phase. Notably, significant healing of the generated cracks in the Li2MnO3·LiMO2 electrode particle occurred during subsequent lithiation on discharge, with this rehealing being principally associated with the solid-solution reaction of the LiMO2 phase. This work reveals that while it is the reduction of lattice size of electrode phases during charge that results in cracking of the Li2MnO3·LiMO2 electrode particle, with the extent of cracking correlated to the magnitude of the size change, crack healing is possible in the reverse solid-solution reaction occurring during discharge. Importantly, it is the phase separation during the two-phase reaction of the LiMO2 phase that prevents the complete healing of the electrode particle, leading to pulverization over extended cycling. This work points to the minimization of behavior leading to phase separation, such as two-phase and oxygen evolution, as a key strategy in preventing capacity fade of the electrode.
中文翻译:

Li2MnO3·LiMO2(M= Li, Ni, Co, Mn) 微球正极容量衰减的起源:AnOperando中子衍射和透射X射线显微镜研究
使用原位中子粉末衍射和透射 X 射线显微镜方法相结合,研究了全电池中 Li2MnO3·LiMO2(M = Li、Ni、Co、Mn)复合正极的容量衰减机制,使相、了解材料在电化学循环过程中的晶体学和形态演变。该电极最初由 73(1) wt% R3̅m LiMO2 组成,其余 27(1) wt% C2/m Li2MnO3 可能作为共生物存在。在手术显微镜观察下,Li2MnO3·LiMO2 电极颗粒中的裂纹是由 LiMO2 相在充电至 4.55 V vs Li(+)/Li 时的固溶反应引发的,并在进一步充电至 4.7 V vs Li( +)/Li 在 LiMO2 相的并发两相反应期间,涉及任何相中最大的晶格变化,以及从 Li2MnO3 相中析出氧。值得注意的是,Li2MnO3·LiMO2 电极颗粒中产生的裂纹在随后的放电锂化过程中发生了显着的愈合,这种再愈合主要与 LiMO2 相的固溶反应有关。这项工作表明,虽然充电过程中电极相晶格尺寸的减小导致 Li2MnO3·LiMO2 电极颗粒开裂,但开裂程度与尺寸变化的幅度相关,相反,裂纹愈合是可能的放电过程中发生的固溶反应。重要的是,LiMO2 相的两相反应过程中的相分离阻止了电极颗粒的完全愈合,长时间循环导致粉化。这项工作指出将导致相分离的行为(例如两相和析氧)最小化,这是防止电极容量衰减的关键策略。
更新日期:2016-07-06
中文翻译:

Li2MnO3·LiMO2(M= Li, Ni, Co, Mn) 微球正极容量衰减的起源:AnOperando中子衍射和透射X射线显微镜研究
使用原位中子粉末衍射和透射 X 射线显微镜方法相结合,研究了全电池中 Li2MnO3·LiMO2(M = Li、Ni、Co、Mn)复合正极的容量衰减机制,使相、了解材料在电化学循环过程中的晶体学和形态演变。该电极最初由 73(1) wt% R3̅m LiMO2 组成,其余 27(1) wt% C2/m Li2MnO3 可能作为共生物存在。在手术显微镜观察下,Li2MnO3·LiMO2 电极颗粒中的裂纹是由 LiMO2 相在充电至 4.55 V vs Li(+)/Li 时的固溶反应引发的,并在进一步充电至 4.7 V vs Li( +)/Li 在 LiMO2 相的并发两相反应期间,涉及任何相中最大的晶格变化,以及从 Li2MnO3 相中析出氧。值得注意的是,Li2MnO3·LiMO2 电极颗粒中产生的裂纹在随后的放电锂化过程中发生了显着的愈合,这种再愈合主要与 LiMO2 相的固溶反应有关。这项工作表明,虽然充电过程中电极相晶格尺寸的减小导致 Li2MnO3·LiMO2 电极颗粒开裂,但开裂程度与尺寸变化的幅度相关,相反,裂纹愈合是可能的放电过程中发生的固溶反应。重要的是,LiMO2 相的两相反应过程中的相分离阻止了电极颗粒的完全愈合,长时间循环导致粉化。这项工作指出将导致相分离的行为(例如两相和析氧)最小化,这是防止电极容量衰减的关键策略。

































 京公网安备 11010802027423号
京公网安备 11010802027423号