当前位置:
X-MOL 学术
›
Mol. Pharmaceutics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
TLR7/8 Agonist-Loaded Nanoparticles Augment NK Cell-Mediated Antibody-Based Cancer Immunotherapy.
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2020-05-08 , DOI: 10.1021/acs.molpharmaceut.0c00271 Hyunjoon Kim 1 , Vidhi Khanna 1 , Tamara A Kucaba 2 , Wenqiu Zhang 1 , Drishti Sehgal 2 , David M Ferguson 3 , Thomas S Griffith 2, 4, 5, 6 , Jayanth Panyam 1, 4
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2020-05-08 , DOI: 10.1021/acs.molpharmaceut.0c00271 Hyunjoon Kim 1 , Vidhi Khanna 1 , Tamara A Kucaba 2 , Wenqiu Zhang 1 , Drishti Sehgal 2 , David M Ferguson 3 , Thomas S Griffith 2, 4, 5, 6 , Jayanth Panyam 1, 4
Affiliation
|
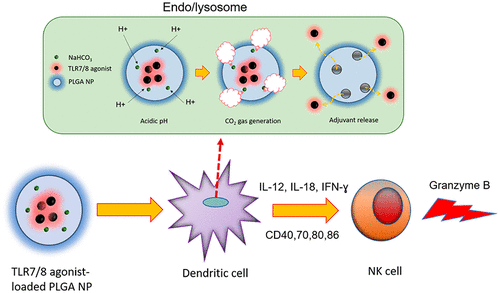
Activated natural killer (NK) cells can kill malignant tumor cells via granule exocytosis and secretion of IFN-γ, a key regulator of the TH1 response. Thus, mobilization of NK cells can augment cancer immunotherapy, particularly when mediated through antibody-dependent cellular cytotoxicity (ADCC). Stimulation of toll-like receptor (TLR)7/8 activity in dendritic cells promotes pro-inflammatory cytokine secretion and costimulatory molecule upregulation, both of which can potentiate NK cell activation. However, currently available TLR7/8 agonists exhibit unfavorable pharmacokinetics, limiting their in vivo efficacy. To enable efficient delivery to antigen-presenting cells, we encapsulated a novel imidazoquinoline-based TLR7/8 agonist in pH-responsive polymeric NPs. Enhanced costimulatory molecule expression on dendritic cells and a stronger pro-inflammatory cytokine response were observed with a NP-encapsulated agonist, compared to that with the soluble form. Treatment with NP-encapsulated agonists resulted in stronger in vivo cytotoxicity and prolonged activation of NK cells compared to that with a soluble agonist. In addition, TLR7/8 agonist-loaded NPs potentiated stronger NK cell degranulation, which resulted in enhanced in vitro and in vivo ADCC mediated by the epidermal growth factor receptor-targeting antibody cetuximab. TLR7/8 agonist-loaded NP treatment significantly enhanced the antitumor efficacy of cetuximab and an anti-HER2/neu antibody in mouse tumor models. Collectively, our data show that a pH-responsive NP-encapsulating TLR7/8 agonist could be used as a potent immunostimulatory adjuvant for antibody-based cancer immunotherapy by promoting NK cell activation.
中文翻译:

TLR7 / 8载有激动剂的纳米颗粒增强了NK细胞介导的基于抗体的癌症免疫治疗。
活化的自然杀伤(NK)细胞可以通过颗粒胞吐作用和TH1反应的关键调节因子IFN-γ的分泌杀死恶性肿瘤细胞。因此,NK细胞的动员可以增强癌症的免疫疗法,尤其是在通过抗体依赖性细胞毒性(ADCC)介导时。刺激树突状细胞中的Toll样受体(TLR)7/8活性可促进促炎性细胞因子分泌和共刺激分子上调,两者均可增强NK细胞的活化。然而,当前可用的TLR7 / 8激动剂表现出不利的药代动力学,限制了它们的体内功效。为了能够有效递送至抗原呈递细胞,我们在pH响应型聚合物NP中封装了一种新型的基于咪唑并喹啉的TLR7 / 8激动剂。与可溶形式相比,用NP包封的激动剂观察到树突状细胞上共刺激分子表达增强,促炎性细胞因子应答更强。与可溶性激动剂相比,用NP封装的激动剂进行治疗可产生更强的体内细胞毒性,并延长NK细胞的活化时间。此外,负载TLR7 / 8激动剂的NP增强了更强的NK细胞脱粒作用,从而增强了体内和体外ADCC由表皮生长因子受体靶向抗体西妥昔单抗介导。载有TLR7 / 8激动剂的NP治疗在小鼠肿瘤模型中显着增强了西妥昔单抗和抗HER2 / neu抗体的抗肿瘤功效。总体而言,我们的数据表明,pH响应NP包裹的TLR7 / 8激动剂可以通过促进NK细胞活化,用作基于抗体的癌症免疫治疗的有效免疫刺激佐剂。
更新日期:2020-05-08
中文翻译:

TLR7 / 8载有激动剂的纳米颗粒增强了NK细胞介导的基于抗体的癌症免疫治疗。
活化的自然杀伤(NK)细胞可以通过颗粒胞吐作用和TH1反应的关键调节因子IFN-γ的分泌杀死恶性肿瘤细胞。因此,NK细胞的动员可以增强癌症的免疫疗法,尤其是在通过抗体依赖性细胞毒性(ADCC)介导时。刺激树突状细胞中的Toll样受体(TLR)7/8活性可促进促炎性细胞因子分泌和共刺激分子上调,两者均可增强NK细胞的活化。然而,当前可用的TLR7 / 8激动剂表现出不利的药代动力学,限制了它们的体内功效。为了能够有效递送至抗原呈递细胞,我们在pH响应型聚合物NP中封装了一种新型的基于咪唑并喹啉的TLR7 / 8激动剂。与可溶形式相比,用NP包封的激动剂观察到树突状细胞上共刺激分子表达增强,促炎性细胞因子应答更强。与可溶性激动剂相比,用NP封装的激动剂进行治疗可产生更强的体内细胞毒性,并延长NK细胞的活化时间。此外,负载TLR7 / 8激动剂的NP增强了更强的NK细胞脱粒作用,从而增强了体内和体外ADCC由表皮生长因子受体靶向抗体西妥昔单抗介导。载有TLR7 / 8激动剂的NP治疗在小鼠肿瘤模型中显着增强了西妥昔单抗和抗HER2 / neu抗体的抗肿瘤功效。总体而言,我们的数据表明,pH响应NP包裹的TLR7 / 8激动剂可以通过促进NK细胞活化,用作基于抗体的癌症免疫治疗的有效免疫刺激佐剂。































 京公网安备 11010802027423号
京公网安备 11010802027423号