当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Chemical Equivalent of Arene Monooxygenases: Dearomative Synthesis of Arene Oxides and Oxepines
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-05-08 , DOI: 10.1021/jacs.0c02724 Zohaib Siddiqi 1 , William C Wertjes 1 , David Sarlah 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-05-08 , DOI: 10.1021/jacs.0c02724 Zohaib Siddiqi 1 , William C Wertjes 1 , David Sarlah 1
Affiliation
|
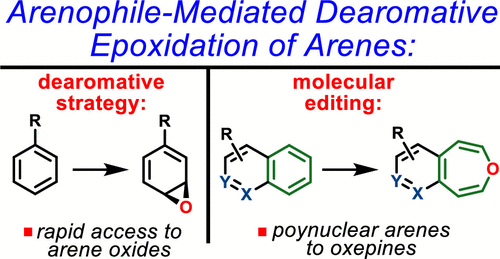
Direct epoxidation of aromatic nuclei by cytochrome P450 monooxygenases is one of the major metabolic pathways of arenes in eukaryotes. The resulting arene oxides serve as versatile precursors to phenols, oxepines, or trans-dihydrodiol-based metabolites. Although such compounds have an important biological and chemical relevance, the lack of methods for their production has hampered access to their utility. Herein, we report a general arenophile-based strategy for the dearomative synthesis of arene oxides. The mildness of this method permits access to sensitive monocyclic arene oxides without any noticeable decomposition to phenols. Moreover, this method enables direct conversion of polycyclic arenes and heteroarenes into the corresponding oxepines. Finally, these studies provided direct connection between simple aromatic precursors and complex small organic molecules via arene oxides and oxepines.
中文翻译:

芳烃单加氧酶的化学当量:芳烃氧化物和 Oxepines 的脱芳基合成
细胞色素 P450 单加氧酶对芳香核的直接环氧化是真核生物中芳烃的主要代谢途径之一。所得的芳烃氧化物可用作酚类、氧杂环庚烷类或基于反式二氢二醇的代谢物的通用前体。尽管此类化合物具有重要的生物学和化学相关性,但由于缺乏其生产方法,阻碍了对其效用的获取。在此,我们报告了一种用于芳烃氧化物脱芳烃合成的基于亲核剂的一般策略。这种方法的温和性允许获得敏感的单环芳烃氧化物,而不会明显分解为酚类。此外,该方法能够将多环芳烃和杂芳烃直接转化为相应的氧杂环庚烷。最后,
更新日期:2020-05-08
中文翻译:

芳烃单加氧酶的化学当量:芳烃氧化物和 Oxepines 的脱芳基合成
细胞色素 P450 单加氧酶对芳香核的直接环氧化是真核生物中芳烃的主要代谢途径之一。所得的芳烃氧化物可用作酚类、氧杂环庚烷类或基于反式二氢二醇的代谢物的通用前体。尽管此类化合物具有重要的生物学和化学相关性,但由于缺乏其生产方法,阻碍了对其效用的获取。在此,我们报告了一种用于芳烃氧化物脱芳烃合成的基于亲核剂的一般策略。这种方法的温和性允许获得敏感的单环芳烃氧化物,而不会明显分解为酚类。此外,该方法能够将多环芳烃和杂芳烃直接转化为相应的氧杂环庚烷。最后,

















































 京公网安备 11010802027423号
京公网安备 11010802027423号