当前位置:
X-MOL 学术
›
Anal. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Development of a Sample-Preparation Workflow for Sulfopeptide Enrichment: From Target Analysis to Challenges in Shotgun Sulfoproteomics.
Analytical Chemistry ( IF 6.7 ) Pub Date : 2020-05-08 , DOI: 10.1021/acs.analchem.0c01342
Anna Laura Capriotti 1 , Andrea Cerrato 1 , Aldo Laganà 1, 2 , Carmela Maria Montone 1 , Susy Piovesana 1 , Riccardo Zenezini Chiozzi 3, 4 , Chiara Cavaliere 1
Analytical Chemistry ( IF 6.7 ) Pub Date : 2020-05-08 , DOI: 10.1021/acs.analchem.0c01342
Anna Laura Capriotti 1 , Andrea Cerrato 1 , Aldo Laganà 1, 2 , Carmela Maria Montone 1 , Susy Piovesana 1 , Riccardo Zenezini Chiozzi 3, 4 , Chiara Cavaliere 1
Affiliation
|
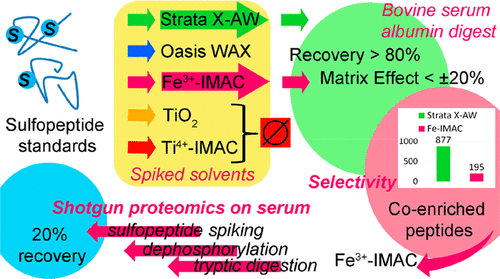
Protein tyrosine O-sulfation is an important post-translational modification, as it has been correlated to inflammation, virus infection, and signal pathways. Nevertheless, methods for the characterization of protein sulfation by sulfopeptide enrichment are currently limited. In this Article, two standard compounds, representative of mono- and disulfated peptides, were used to compare the enrichment capabilities of five sorbent materials: two commercial weak anion-exchange mixed-mode sorbents (Strata X-AW and Oasis WAX) and three phosphopeptide enrichment materials based on affinity chromatography to either immobilized metals (IMAC) or metal oxides, i.e., Fe3+, TiO2, or Ti4+. The sulfopeptides were analyzed by ultrahigh-performance liquid chromatography (UHPLC) multiple-reaction monitoring analysis and were stable under all the tested experimental conditions. Recoveries of the enrichment step from spiked bovine serum albumin digests were >80% for the commercial Fe-IMAC kit and the Strata X-AW sorbent. Shotgun proteomics was used on the same samples to evaluate the selectivity, calculated as the number of coenriched peptides, and it was found to be better for the Fe-IMAC kit. Therefore, the Fe-IMAC protocol was embedded in a shotgun-proteomics workflow and applied to serum spiked with the sulfopeptides before protein dephosphorylation and digestion. The recovery of the entire analytical workflow was 20%, which was compatible with previous data on TiO2 phosphopeptide enrichment. Despite the potential, no sulfopeptide was confidently identified in serum digests by conventional shotgun proteomics, probably due to very low abundance of native sulfoproteins, poor ionization efficiency of sulfopeptides in the positive mode, and lack of unambiguous sulfopeptide identification by bioinformatics software. In this context, the use of negative-ionization mode with high-resolution mass spectrometry appeared promising to improve the sensibility and allow sulfopeptide identification in complex samples.
中文翻译:

磺肽富集的样品制备工作流程的开发:从靶标分析到Shot弹枪仿制药的挑战。
蛋白质酪氨酸的O-硫酸化是重要的翻译后修饰,因为它与炎症,病毒感染和信号通路相关。然而,通过硫肽富集表征蛋白质硫酸化的方法目前受到限制。在本文中,使用两种代表单硫酸盐和二硫酸盐肽的标准化合物来比较五种吸附剂材料的富集能力:两种市售弱阴离子交换混合模式吸附剂(Strata X-AW和Oasis WAX)和三种磷酸肽基于亲和色谱的固定化金属(IMAC)或金属氧化物(例如,Fe 3+,TiO 2或Ti 4+)的富集材料。硫肽通过超高效液相色谱(UHPLC)多重反应监测分析进行分析,并且在所有测试的实验条件下均稳定。对于商业化的Fe-IMAC试剂盒和Strata X-AW吸附剂,加标的牛血清白蛋白消化液富集步骤的回收率> 80%。在相同的样品上使用弹枪蛋白质组学来评估选择性(以共富集的肽数计算),发现Fe-IMAC试剂盒更好。因此,Fe-IMAC协议被嵌入到gun弹枪蛋白质组学工作流程中,并应用于在蛋白质去磷酸化和消化之前加有磺肽的血清。整个分析工作流程的回收率为20%,与先前有关TiO 2的数据兼容磷酸肽富集。尽管具有这种潜力,但常规shot弹枪蛋白质组学在血清消化液中仍未确定地鉴定出硫肽,这可能是由于天然硫蛋白的丰度非常低,在正模式下硫肽的电离效率很低,以及生物信息学软件无法明确鉴定硫肽。在这种情况下,使用负离子电离模式和高分辨率质谱技术似乎有望改善灵敏度,并允许在复杂样品中鉴定磺肽。
更新日期:2020-05-08
中文翻译:

磺肽富集的样品制备工作流程的开发:从靶标分析到Shot弹枪仿制药的挑战。
蛋白质酪氨酸的O-硫酸化是重要的翻译后修饰,因为它与炎症,病毒感染和信号通路相关。然而,通过硫肽富集表征蛋白质硫酸化的方法目前受到限制。在本文中,使用两种代表单硫酸盐和二硫酸盐肽的标准化合物来比较五种吸附剂材料的富集能力:两种市售弱阴离子交换混合模式吸附剂(Strata X-AW和Oasis WAX)和三种磷酸肽基于亲和色谱的固定化金属(IMAC)或金属氧化物(例如,Fe 3+,TiO 2或Ti 4+)的富集材料。硫肽通过超高效液相色谱(UHPLC)多重反应监测分析进行分析,并且在所有测试的实验条件下均稳定。对于商业化的Fe-IMAC试剂盒和Strata X-AW吸附剂,加标的牛血清白蛋白消化液富集步骤的回收率> 80%。在相同的样品上使用弹枪蛋白质组学来评估选择性(以共富集的肽数计算),发现Fe-IMAC试剂盒更好。因此,Fe-IMAC协议被嵌入到gun弹枪蛋白质组学工作流程中,并应用于在蛋白质去磷酸化和消化之前加有磺肽的血清。整个分析工作流程的回收率为20%,与先前有关TiO 2的数据兼容磷酸肽富集。尽管具有这种潜力,但常规shot弹枪蛋白质组学在血清消化液中仍未确定地鉴定出硫肽,这可能是由于天然硫蛋白的丰度非常低,在正模式下硫肽的电离效率很低,以及生物信息学软件无法明确鉴定硫肽。在这种情况下,使用负离子电离模式和高分辨率质谱技术似乎有望改善灵敏度,并允许在复杂样品中鉴定磺肽。

































 京公网安备 11010802027423号
京公网安备 11010802027423号