Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Theoretical Studies on the Stability and Reactivity of the Metal-Doped CeO2(100) Surface: Toward H2 Dissociation and Oxygen Vacancy Formation.
Langmuir ( IF 3.7 ) Pub Date : 2020-05-07 , DOI: 10.1021/acs.langmuir.0c00644 Wei Zhang 1 , Min Pu 1 , Ming Lei 1
Langmuir ( IF 3.7 ) Pub Date : 2020-05-07 , DOI: 10.1021/acs.langmuir.0c00644 Wei Zhang 1 , Min Pu 1 , Ming Lei 1
Affiliation
|
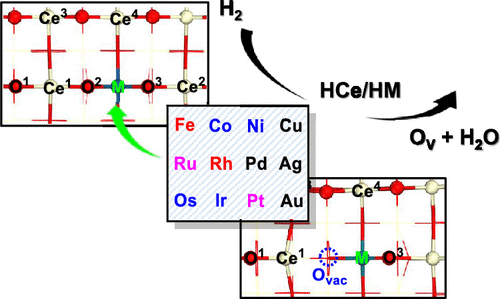
Surface doping is a common method to improve the performance of nanostructured materials. Different dopants will affect the structure and catalytic reactivity of the support. For a comprehensive understanding of the doping effects of metals doped into CeO2, we conducted density functional theory (DFT) studies on the stabilities and geometry structures of transition-metal atoms (M = Fe, Co, Ni, Cu; Ru, Rh, Pd, Ag; Os, Ir, Pt, Au) doped into CeO2(111), (110), and (100) surfaces. Moreover, the reactivity for H2 dissociation and oxygen vacancy formation are systematically investigated on M-doped CeO2(100) surfaces. The greater the binding energies of doped M atoms on the CeO2 surface, the more difficult the formation of oxygen vacancies. The doped Co and Ir atoms do not directly participate in H2 activation but serve as a promoter to make the H–H bond to break easily. The Cu, Ru, Pd, Ag, Pt, and Au atoms could act as the catalytically active center for H2 dissociation and greatly reduce the activation energy barrier. Besides, it is easier to generate H2O (WM) and a surface oxygen vacancy from the intermediate H2M/H4M than from H3M/H5M, which is related to the acid–base interaction between HCe/M* and HO* in H2M/H4M. This work could provide theoretical insights into the atomic structure characteristics of the transition-metal-doped CeO2(100) surface and give ideas for the design of hydrogenation catalysts.
中文翻译:

掺杂金属的CeO2(100)表面的稳定性和反应性的理论研究:H2离解和氧空位的形成。
表面掺杂是提高纳米结构材料性能的常用方法。不同的掺杂剂将影响载体的结构和催化反应性。为了全面了解掺杂到CeO 2中的金属的掺杂效果,我们对过渡金属原子(M = Fe,Co,Ni,Cu,Ru,Rh,将Pd,Ag,Os,Ir,Pt,Au掺杂到CeO 2(111),(110)和(100)表面中。此外,系统地研究了M掺杂的CeO 2(100)表面上H 2离解和氧空位形成的反应性。CeO 2上掺杂的M原子的结合能越大表面上,氧空位的形成越困难。掺杂的Co和Ir原子不直接参与H 2活化,但起促进剂的作用,使H–H键容易断裂。Cu,Ru,Pd,Ag,Pt和Au原子可以充当H 2离解的催化活性中心,并大大降低活化能垒。此外,从中间H2 M / H4 M生成H 2 O(W M)和表面氧空位比从H3 M / H5 M生成更容易,这与H Ce / M之间的酸碱相互作用有关。和HO *在H2 M / H4 M中。这项工作可以为过渡金属掺杂CeO 2(100)表面的原子结构特征提供理论见解,并为氢化催化剂的设计提供思路。
更新日期:2020-05-07
中文翻译:

掺杂金属的CeO2(100)表面的稳定性和反应性的理论研究:H2离解和氧空位的形成。
表面掺杂是提高纳米结构材料性能的常用方法。不同的掺杂剂将影响载体的结构和催化反应性。为了全面了解掺杂到CeO 2中的金属的掺杂效果,我们对过渡金属原子(M = Fe,Co,Ni,Cu,Ru,Rh,将Pd,Ag,Os,Ir,Pt,Au掺杂到CeO 2(111),(110)和(100)表面中。此外,系统地研究了M掺杂的CeO 2(100)表面上H 2离解和氧空位形成的反应性。CeO 2上掺杂的M原子的结合能越大表面上,氧空位的形成越困难。掺杂的Co和Ir原子不直接参与H 2活化,但起促进剂的作用,使H–H键容易断裂。Cu,Ru,Pd,Ag,Pt和Au原子可以充当H 2离解的催化活性中心,并大大降低活化能垒。此外,从中间H2 M / H4 M生成H 2 O(W M)和表面氧空位比从H3 M / H5 M生成更容易,这与H Ce / M之间的酸碱相互作用有关。和HO *在H2 M / H4 M中。这项工作可以为过渡金属掺杂CeO 2(100)表面的原子结构特征提供理论见解,并为氢化催化剂的设计提供思路。































 京公网安备 11010802027423号
京公网安备 11010802027423号