当前位置:
X-MOL 学术
›
ChemistrySelect
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Structure Comparison of Ni(II) Complexes Supported by PNCNP and POCOP Pincer Ligands
ChemistrySelect ( IF 1.9 ) Pub Date : 2020-05-05 , DOI: 10.1002/slct.202001413 Fei Fang 1 , Jiarui Chang 1 , Jiaxin Kang 1 , Jie Zhang 1 , Shujun Li 1 , Xuenian Chen 1, 2
ChemistrySelect ( IF 1.9 ) Pub Date : 2020-05-05 , DOI: 10.1002/slct.202001413 Fei Fang 1 , Jiarui Chang 1 , Jiaxin Kang 1 , Jie Zhang 1 , Shujun Li 1 , Xuenian Chen 1, 2
Affiliation
|
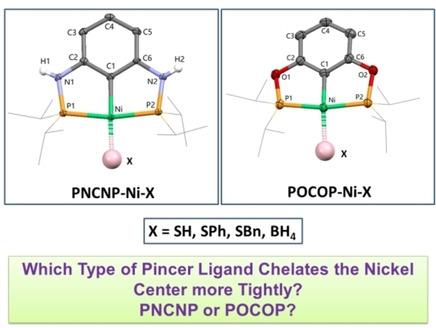
In order to compare the structure and property of the same metal center supported by different types of pincer ligands, a series of Ni(II) pincer complexes, [2,6‐(iPr2PNH)2C6H3]NiX (X=SH, 1 a; SPh, 1 b; SBn, 1 c; BH4, 1 d), were synthesized based on m‐(iPr2PNH)2C6H4 (PNCNP) and fully characterized by multinuclear NMR, FTIR, X‐ray crystallography and elemental analysis. The structures of complexes 1 a–d were compared with those of the corresponding Ni(II) complexes supported by a bis(phosphinite) (POCOP) pincer ligand, [2,6‐(iPr2PO)2C6H3]NiX (X=SH, 2 a; SPh, 2 b; SBn, 2 c; BH4, 2 d). It was found that the Ni‐Cipso and Ni−S bond lengths and the Ni⋅⋅⋅B distance in complexes 1 a–d are comparable to those in complexes 2 a–d; however, the Ni−P bond lengths in complexes 1 a–d are longer than those in complexes 2 a–d. As a result, the nickel center is less tightly chelated by the PNCNP backbone compared with the POCOP framework. Natural population analysis (NPA) of complexes 1 a, 2 a, 1 b and 2 b indicated that the nickel center supported by a PNCNP pincer ligand is less electron‐rich. Cyclic voltammetry study of 1 a and 2 a showed that 2 a is easier to be oxidized.
中文翻译:

PNCNP和POCOP夹钳配体支持的Ni(II)配合物的结构比较
为了比较不同类型的钳夹配体支持的同一金属中心的结构和性能,我们使用了一系列Ni(II)钳夹配合物[2,6‐(i Pr 2 PNH)2 C 6 H 3 ] NiX( X = SH,1 ; SPh上,1b中; SBn的,图1c ; BH 4,1 d)的基础上,合成了米- (我镨2 PNH)2 C ^ 6 ħ 4(PNCNP),并通过多核NMR完全表征,FTIR,X射线晶体学和元素分析。配合物的结构1 a –将d与双(次膦酸酯)(POCOP)钳配体[2,6-‐(i Pr 2 PO)2 C 6 H 3 ] NiX(X = SH,2一个; SPh上,2 b ; SBn的,图2c ; BH 4,2 d)。结果发现,在Ni-C本位和Ni-S键长和在复合物中的Ni⋅⋅⋅B距离1 - d比得上那些配合物2 - d ; 但是,配合物1 a – d中的Ni-P键长比络合物2 a – d中的那些更长。因此,与POCOP框架相比,PNCNP主链不会更紧密地螯合镍中心。复合物的天然人口分析(NPA)1,2,图1b和2b中表示,镍中心由PNCNP钳形支持配体是富电子的较少。1 a和2 a的循环伏安法研究表明2 a更容易被氧化。
更新日期:2020-05-05
中文翻译:

PNCNP和POCOP夹钳配体支持的Ni(II)配合物的结构比较
为了比较不同类型的钳夹配体支持的同一金属中心的结构和性能,我们使用了一系列Ni(II)钳夹配合物[2,6‐(i Pr 2 PNH)2 C 6 H 3 ] NiX( X = SH,1 ; SPh上,1b中; SBn的,图1c ; BH 4,1 d)的基础上,合成了米- (我镨2 PNH)2 C ^ 6 ħ 4(PNCNP),并通过多核NMR完全表征,FTIR,X射线晶体学和元素分析。配合物的结构1 a –将d与双(次膦酸酯)(POCOP)钳配体[2,6-‐(i Pr 2 PO)2 C 6 H 3 ] NiX(X = SH,2一个; SPh上,2 b ; SBn的,图2c ; BH 4,2 d)。结果发现,在Ni-C本位和Ni-S键长和在复合物中的Ni⋅⋅⋅B距离1 - d比得上那些配合物2 - d ; 但是,配合物1 a – d中的Ni-P键长比络合物2 a – d中的那些更长。因此,与POCOP框架相比,PNCNP主链不会更紧密地螯合镍中心。复合物的天然人口分析(NPA)1,2,图1b和2b中表示,镍中心由PNCNP钳形支持配体是富电子的较少。1 a和2 a的循环伏安法研究表明2 a更容易被氧化。






























 京公网安备 11010802027423号
京公网安备 11010802027423号