当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The Role of Fe Species on NiOOH in Oxygen Evolution Reactions
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-05-05 , DOI: 10.1021/acscatal.0c00304 Yecheng Zhou 1, 2 , Núria López 2
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-05-05 , DOI: 10.1021/acscatal.0c00304 Yecheng Zhou 1, 2 , Núria López 2
Affiliation
|
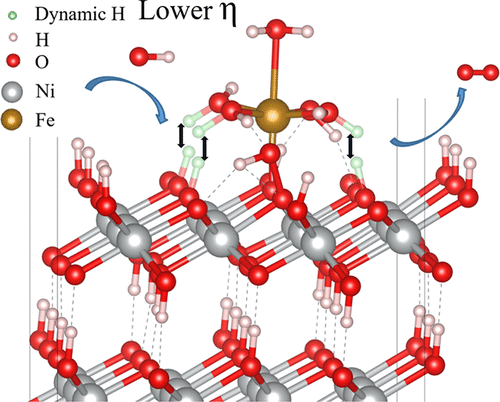
The Pourbaix diagram of Ni electrodes under reaction conditions presents several metastable NiOxHy phases and Fe doping enlarges the stability area of oxyhydroxo species. For the Ni-only phase, water adsorption and intercalation can significantly lower both the surface and interface energies, and even introduce “negative surface energy”. Thus, water can exfoliate layers, leading to Fe ion adsorption on inner layers, as demonstrated by ab initio molecular dynamics. These single atoms have been carefully speciated (i.e., initially prepared as Fe2+ and Fe3+) and proton coupled electron transfer between the H2O–Fe and lattice oxygen ions has been observed in all ab initio molecular dynamics simulations, which is attributed to the Fe incorporation, since no proton coupled electron transfer occurs under free water conditions. Furthermore, 15 possible oxygen evolution reaction mechanisms near Fe ions show that the main active species corresponds to the Ni2+, which is reduced from Ni3+ via H transfer when a Fe2+ iron adsorbs nearby, and the overpotential can be significantly reduced to 0.23 V.
中文翻译:

Fe物种对NiOOH在析氧反应中的作用
在反应条件下,Ni电极的Pourbaix图显示了几个亚稳态的NiO x H y相,Fe的掺杂扩大了羟基氧化物的稳定性。对于纯镍相,水的吸附和嵌入可以显着降低表面能和界面能,甚至引入“负表面能”。因此,水会剥落各层,导致铁离子吸附在内层上,如从头算分子动力学所证实的。这些单原子经过仔细指定(即最初制备为Fe 2+和Fe 3+),并且质子耦合的电子在H 2之间转移在所有的从头算分子动力学模拟中都观察到了O–Fe和晶格氧离子,这归因于Fe的掺入,因为在自由水条件下没有质子耦合电子转移发生。此外,Fe离子附近的15种可能的氧释放反应机理表明,主要活性物质对应于Ni 2+,当Fe 2+铁吸附在附近时,其通过H转移从Ni 3+还原,并且可以显着降低过电势至0.23 V
更新日期:2020-05-05
中文翻译:

Fe物种对NiOOH在析氧反应中的作用
在反应条件下,Ni电极的Pourbaix图显示了几个亚稳态的NiO x H y相,Fe的掺杂扩大了羟基氧化物的稳定性。对于纯镍相,水的吸附和嵌入可以显着降低表面能和界面能,甚至引入“负表面能”。因此,水会剥落各层,导致铁离子吸附在内层上,如从头算分子动力学所证实的。这些单原子经过仔细指定(即最初制备为Fe 2+和Fe 3+),并且质子耦合的电子在H 2之间转移在所有的从头算分子动力学模拟中都观察到了O–Fe和晶格氧离子,这归因于Fe的掺入,因为在自由水条件下没有质子耦合电子转移发生。此外,Fe离子附近的15种可能的氧释放反应机理表明,主要活性物质对应于Ni 2+,当Fe 2+铁吸附在附近时,其通过H转移从Ni 3+还原,并且可以显着降低过电势至0.23 V





















































 京公网安备 11010802027423号
京公网安备 11010802027423号